0246
MRI-based deep learning radiomics can predict HER2 expression and disease-free survival in breast cancer1Guangzhou First People's Hospital, Guangzhou, China, 2Shenzhen University, Shenzhen, China, 3Philips Healthcare, Guangzhou, China, 4Guangdong Women and Children Hospital, Guangzhou, China
Synopsis
Keywords: Radiomics, Breast, HER2 expressing; MRI; Deep learning; Prognosis
To the best of our knowledge, our study is the first to non-invasively assess human epidermal growth factor receptor 2 (HER2) status, especially HER2-low-positive status in breast cancer. In this study, a deep learning radiomics (DLR) model based on contrast-enhanced MRI was constructed and showed high and stable performance in predicting HER2 status in both the training and validation cohorts, and the predicted status was an independently significant predictor of disease-free survival (DFS) in HER2-low-positive/HER2-zero breast cancers. The DLR model showed prospects as a computer-aided diagnostic tool to help more accurately identify HER2-low-positive breast cancers, thereby guiding patient treatment strategies.Introduction
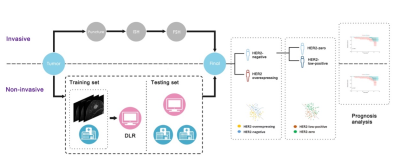
Recent literature has focused on human epidermal growth factor receptor 2 (HER2)-low-positive breast cancer as a novel predictor for prognosis and for developing novel therapeutic targets.[1; 2] A recent study published in Lancet Oncology found that hormone receptor status, tumour proliferation, grading, and response to neoadjuvant chemotherapy were significantly different between HER2-low-positive breast cancer and HER2-zero breast cancer.[3] Currently, HER2 expression is determined by invasively sampling tissue specimens. However, sampling bias can exist due to the heterogeneous nature of tumours, and the assays are invasive and may fail to yield actionable results due to insufficient tissue quantity or quality. Therefore, it is important to develop a non-invasive HER2 status prediction method. Deep learning radiomics (DLR), a newly developed method, can provide quantitative and high-throughput features from medical images by supervised learning, which could be used to analyze MRI and has shown excellent performance in predicting tumor biological information.[4; 5] Hence, the purpose of this study is to develop an MR-based DLR method, to non-invasively assess HER2 status in breast cancer, especially HER2-low-positive status, and to investigate the effect of the prediction score on the prognosis of breast cancer. Fig. 1 shows an overview of our study.Methods
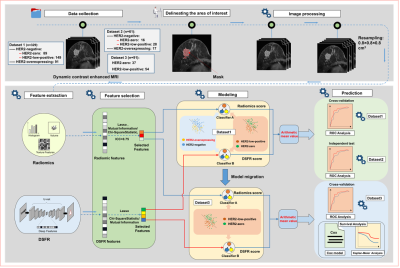
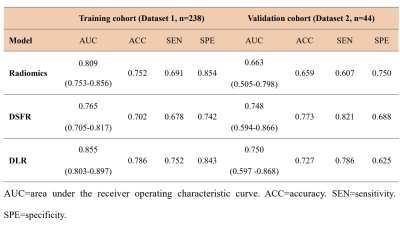
A total of 481 patients with breast cancer who underwent pre-operative MRI were recruited from two institutions (Guangzhou First People’s Hospital and Guangdong Women and Children Hospital). All patients underwent IHC and FISH to determine their HER2 status. Two experienced radiologists who were blinded to the histopathological results contoured the region of interest (ROI) of tumour on the MRI. Traditional radiomics features and deep semantic segmentation feature-based radiomics (DSFR) features were extracted from segmented tumours, and averaging the output probabilities of the two models were used to select HER-2-related features. Finally, a contrast-enhanced MRI-based DLR model was constructed to non-invasively assess HER2 status in breast cancer, especially HER2-low-positive status. We obtained the receiver operating characteristic (ROC) curve according to the prediction results and calculated the receiver operating characteristic curve (AUC) to assess the predictive performance of the constructed models. With the optimal threshold determined by ROC analysis, the accuracy (ACC), sensitivity (SEN), and specificity (SPE) of the prediction models were calculated. The workflow of the DLR model is shown in Fig. 2. DFS was estimated by using the Kaplan-Meier method, and the log-rank test was employed to compare the DFS difference between HER2-low-positive and HER2-zero patients. To further evaluate the prognostic value of HER2 status, a multivariate Cox proportional hazard model was constructed by using the Akaike information criterion (AIC) as the stopping rule to determine the association of the model prediction score and clinicopathological variables with DFS. The C-index between the predicted probability and actual outcome was calculated to evaluate the predictive ability of the model.Results
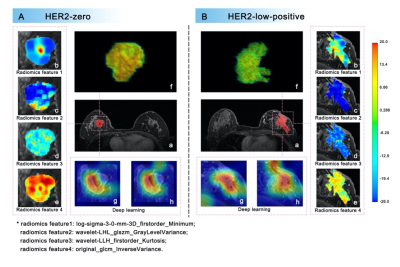
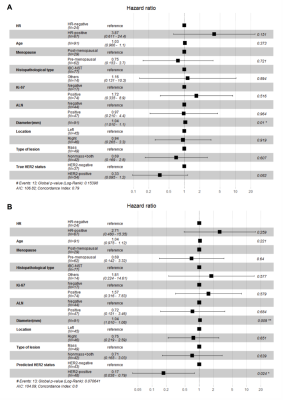
First, the DLR model distinguished between HER2-negative and HER2-overexpressing patients with AUCs of 0.868 (95% CI, 0.827-0.903) in the training cohort and 0.763 (95% CI, 0.637-0.863) in the validation cohort. Further, the DLR model distinguished between HER2-low-positive and HER2-zero patients with AUCs of 0.855 (95% CI, 0.803-0.897) in the training cohort and 0.750 (95% CI, 0.597-0.868) in the validation cohort (Table 1). In this study, four of the top 10 radiomics features were selected to visually show the difference between HER2-low-positive and HER2-zero breast cancer, and visualized deep learning was also visually demonstrated (seen in Fig. 3). Significantly better DFS was found in HER2-low-positive patients than in HER2-zero patients. Cox regression analysis showed that the DLR model prediction score (hazard ratio, 0.175; 95% CI, 0.038-0.792; P=0.024) and lesion size (hazard ratio, 1.043; 95% CI, 1.010-1.077; P=0.009) were significant independent predictors of DFS (seen in Fig. 4).Discussion
Radiomics approaches have been used to detect and characterize HER2 status in breast cancer.[6; 7] However, most of them focused on distinguishing between HER2-negative and HER2-overexpressing tumours. Besides, these radiomics model studies have not demonstrated high performance. Recently, an emerging trend in medical image analysis has been to combine a radiomics framework with a deep learning model to achieve higher performance. Compared to the radiomics method, the DLR model in our study achieved the highest AUCs, indicating the excellent performance of the integrated DLR approach. In this study, we found a difference in DFS between HER2-low-positive and HER2-zero tumours, which was consistent with that reported by Denkert et al.[3] Cox regression analysis further showed that the HER2 status predicted by the DLR model was an independently significant factor in predicting DFS. Our results not only demonstrated the clinical significance of DLR in distinguishing HER2-low-positive from HER2-negative breast cancer but also verified the DLR model’s predictive score as an important biomarker for the risk stratification of DFS.Conclusion
We successfully constructed a DLR model based on enhanced MR to non-invasively evaluate HER2 status, particularly HER2-low-positive, and further showed prospects for predicting DFS in breast cancer.Acknowledgements
No acknowledgement found.References
[1] Banerji U, van Herpen C, Saura C et al (2019) Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. The Lancet Oncology 20:1124-1135
[2] Modi S, Park H, Murthy R et al (2020) Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 38:1887-1896
[3] Denkert C, Seither F, Schneeweiss A et al (2021) Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. The Lancet Oncology. 10.1016/s1470-2045(21)00301-6
[4] Wang K, Lu X, Zhou H et al (2019) Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut 68:729-741
[5] Gillies R, Kinahan P, Hricak H (2016) Radiomics: Images Are More than Pictures, They Are Data. Radiology 278:563-577
[6] Zhou J, Tan H, Li W et al (2020) Radiomics Signatures Based on Multiparametric MRI for the Preoperative Prediction of the HER2 Status of Patients with Breast Cancer. Academic radiology. 10.1016/j.acra.2020.05.040
[7] Bitencourt A, Gibbs P, Rossi Saccarelli C et al (2020) MRI-based machine learning radiomics can predict HER2 expression level and pathologic response after neoadjuvant therapy in HER2 overexpressing breast cancer. EBioMedicine 61:103042
Figures