0244
Novel LGE Myocardial Scar Burden Signatures: A Novel Concept for Comprehensive Scar Quantification in Myocardial Infarction1Northwestern University, Chicago, IL, United States
Synopsis
Keywords: Radiomics, Cardiomyopathy, heart, cardiovascular, LGE, scar, Myocardial Infarction
There remains no standard method for quantification of myocardial infarction (MI) from LGE images. Current methods lack reproducibility as they rely on manual or threshold-based scar segmentation. Notably, these methods only quantify scar volume/percentage but ignore the impact of myocardial LGE distribution pattern. Here, we propose a novel threshold-free concept that comprehensively quantifies scar burden in terms of both scar extent and the unique myocardial LGE distribution pattern: LGE Scar Burden Signatures. We demonstrated our technique’s strong correlation to scar extent, its independent association with serum biomarker of myocardial injury (Troponin), and reduced ejection fraction, independent of Scar percentage.
Purpose
Late gadolinium enhancement (LGE) MRI has emerged as a clinical standard for the evaluation of myocardial infarction (MI). Nevertheless, there is no established standard method for quantification of MI from LGE images. Current methods rely on time-consuming manual segmentation or threshold-based identification of scarred regions that lack standardization and reproducibility1. Importantly, current methods rely primarily on scar volume/percentage to quantify MI. However, scar percentage ignores LGE distribution pattern in the myocardium and its potential association with severity of scar burden on cardiac function. Here we propose a novel concept that uniquely quantifies both myocardial scar extent and distribution pattern simultaneously and is threshold-free for a comprehensive quantification of MI: LGE Scar Burden Signatures. This concept probabilistically encodes the unique LGE profile (signature) from multi-million LGE intensity co-disparities per patient. This study aimed at evaluating the proposed threshold-free scar burden signatures in terms of 1) the feasibility for differentiating MI patients versus non-MI patients. 2) correlation with scar percentage. 3) independent association with Troponin: a serum biomarker of myocardial injury. 4) association with cardiac function assessed by ejection fraction (EF).Methods
We analyzed multi-slice LV LGE images in a cohort of 100 patients from the publicly available EMIDEC database 2. The cohort is composed of 67 confirmed MI patients (56 Males, age: 58±12yrs) and 33 non-MI patients (8 Males, age:66±13yrs, p for age <0.001) 3. LGE images were accompanied by segmentation of myocardial wall and manually segmented regions of scar and microvascular obstruction (MVO) for scar and MVO percentage quantification and included clinical data of Troponin and ejection fraction (EF).Probabilistic LGE Myocardial Scar Burden Signatures: The stages of our proposed method are presented in Fig. 1 and illustrated in Fig. 2:
Stage I computes the unique cross-disparity signature profile between the LGE myocardial wall and blood pool by encoding the profile as probability density function (pdf) from ~10 million intensity co-disparity associations. Given the blood pool is bright, the method is designed mathematically to force higher disparities on the right side of the pdf i.e., post-peak, and lower disparity (normal tissue) on the left side of the pdf peak.
Stage II computes an auto co-disparity signature of myocardial wall LGE to encode the unique composition of LGE distribution pattern in the entire myocardial wall, including encoding of scar/tissue heterogeneity.
Stage III computes the novel threshold-free scar burden signature index (SBSI) that encodes both scar/tissue extent and LGE distribution patterns simultaneously based on probability density distribution differences of LGE scar signature profiles from Stages I and II.
Association to scar percentage, myocardial injury, and cardiac function: To assess the potential of SBSI as a comprehensive measure of scar burden in MI, we tested its association with scar and MVO percentages and association to Troponin level – as an imaging-independent serum marker of MI, and with cardiac function by EF.
Results
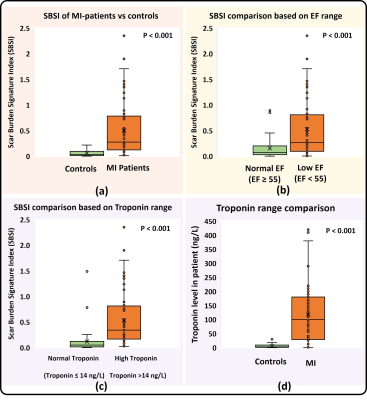
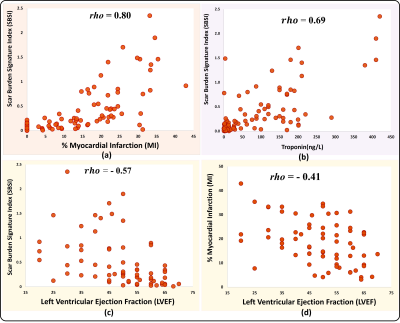
SBSI computation was successful in all 100 patients by encoding 17 ± 11 million LGE pairwise co-disparities per patient. Fig. 3.a demonstrates examples of high and low SBSI burden scans. Fig. 3.b shows two MI patients with the same scar percentage but ~3.5 times difference in SBSI, which agrees with Troponin levels of ~2.5-fold difference. Fig. 4.a show significantly higher SBSI in MI patients versus controls (p<0.001). SBSI index of scar burden was significantly higher (p<0.001) in patients with elevated Troponin than patients with normal Troponin (Fig. 4.c) and in patients with reduced EF (p<0.001) than patients with normal EF (Fig. 4.b). SBSI showed a high correlation with scar extent as measured by scar percentage (Spearman rho=0.80) and moderate correlation to MVO (rho=0.46). SBSI was highly correlated with Troponin biomarker (rho=0.69) (Fig. 5). In MI patients, SBSI showed higher inverse correlation vs. EF (rho=-0.57) than scar percentage vs. EF (rho=-0.40) and MVO percentage vs. EF (rho=0.02). In multivariate regression, SBSI was associated with Troponin (β =71.84 ng/L, R-squared=0.62, p<0.001) and with EF (β=-9.3%, R-squared=0.14, p=0.01) independent of scar percentage, MVO percentage, and age.Discussion
Here, we propose the first threshold-free method for comprehensive scar burden quantification that does not require segmentation of scar regions. By probabilistic analysis of multi-million LGE co-disparities, the proposed Scar Burden Signature Index (SBSI) mathematical definition comprehensively encodes myocardial scar burden in terms of both the scar extent and unique LGE distribution pattern in the myocardial wall. SBSI is a standardized continuous index, inherently normalized to wall volume (using pdf), with higher values indicating higher scar burden.Conclusions
This study demonstrated the feasibility of a novel scar burden signature concept encoding both myocardial scar extent and unique LGE distribution for comprehensive threshold-free scar quantification from LGE in MI patients. The proposed novel SBSI index of scar burden showed a strong correlation with scar percentage and a significant association with Troponin and EF independent of scar, MVO percentages, and age. SBSI showed a higher correlation with EF than scar percentage with EF. Therefore, the results indicate a potential added value of the proposed novel SBSI index over scar percentage as a comprehensive measure of myocardial scar burden in MI. In future, we will assess potential prognostic value of SBSI in different MI types and arrhythmia patients.Acknowledgements
No acknowledgement found.References
[1] Gräni C, et al. Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis, Journal of Cardiovascular Magnetic Resonance, 2019.[2] Lalande, Alain, et al. Emidec: A Database Usable for the Automatic Evaluation of Myocardial Infarction from Delayed-Enhancement Cardiac MRI, data, 2020, 2306-5729.
[3] Alain Lalande, et. Deep learning methods for automatic evaluation of delayed enhancement-MRI. The results of the EMIDEC challenge, Medical Image Analysis, 2022.
Figures

Fig.1 Summary of the three stages of the proposed probabilistic scar signature algorithm to quantify scar in myocardial infarction from LGE MRI.
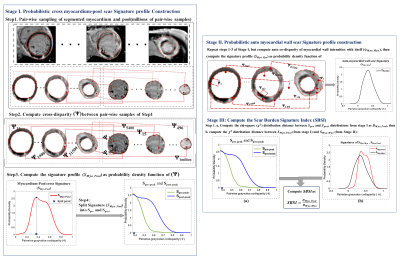
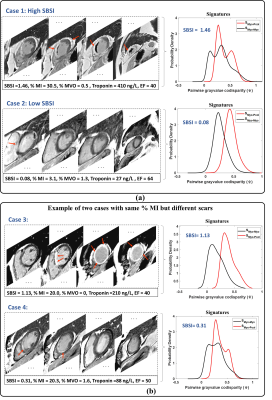
Fig.3a Example cases from the studied AF patients present with high and low SBSI values presented with few of the corresponding LGE slices, and their corresponding scar signature profiles. Fig.3b shows two cases with same MI percentage which is obviously incorrect and verified visually and also by biomarkers.