0222
Multi-Echo EPI Increases Sensitivity to BOLD Activation in the Olfactory Network Compared to Single-Echo EPI1Department of Bioengineering, School of Engineering and Applied Science, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Psychology, School of Arts and Science, University of Pennsylvania, Philadelphia, PA, United States, 4Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Keywords: Brain Connectivity, fMRI, Multi-echo EPI
The human olfactory network presents technical challenges for functional MRI due to its location in regions of high static susceptibility. We developed a novel multi-echo EPI (ME-EPI) protocol, optimized for olfactory regions, and compared this protocol to a conventional single-echo EPI (1E-EPI). We show that the optimized ME-EPI increases sensitivity to BOLD activation response for olfactory regions and reduces the numbers of subjects required to detect significant group effects.Introduction
The human olfactory system is challenging to study using BOLD fMRI because olfactory regions (orbitofrontal cortex, piriform cortex, and amygdala) are near areas of high static susceptibility, introducing artifacts in gradient echo EPI and affecting the optimal echo time (TE) for detecting BOLD effects1. The sensitivity of olfactory fMRI is also degraded by respiratory artifact2.Multi-echo EPI (ME-EPI) has shown improved BOLD sensitivity, both by sampling across a range of TEs and enabling noise reduction based on signal modeling3,4. We conducted an olfactory discrimination task to compare a ME-EPI acquisition optimized for olfactory regions with conventional single-echo EPI (1E-EPI).
Method
Subjects16 subjects (10 women; mean age 26 years) were recruited and provided informed consent. Images were acquired in a 3T scanner (MAGNETOM Prisma, Siemens Healthineers) using the 64-channel head coil.
Experimental Paradigm and Stimuli
Stimuli consisted of two odorants, lemon oil extract (12.3% v/v) and benzaldehyde (0.42% v/v), diluted in mineral oil to produce lemon and almond scents, with pure mineral oil as a control stimulus. fMRI sessions consisted of 6 runs, each containing 24 trials, equally distributed among the three odorants (Figure 1). 3 runs of ME-EPI were interleaved with 3 runs of 1E-EPI. Run-order was counterbalanced across subjects.
Data Acquisition and Preprocessing
T1-weighted structural images were acquired at 1mm resolution using ME-MPRAGE. 1E-EPI and ME-EPI fMRI data were acquired using the sequence parameters shown in Table 1 with an oblique orientation of 25° to the anterior commissure-posterior commissure line (rostral > caudal). Breathing was recorded using a respiratory belt.
Images were preprocessed using fMRIPrep 21.0.0 and registered to MNI standard space before further processing.
ME-EPI data were optimally combined given the estimated T2*5. TE-dependency analysis pipeline was performed for denoising6.
fMRI Processing
fMRI data were analyzed in SPM12 using a general linear model to estimate the main effects of the odorants with the following nuisance regressors: 24 movement parameters, breathing trace, trial-by-trial sniff volume, and duration convolved with HRF. Each experimental condition (control, lemon, and benzaldehyde) was modeled independently, as were multi-echo and single-echo acquisitions.
Two contrasts (lemon > control, benzaldehyde > control) were computed for each subject using one-way t-tests. The group-level analysis was performed using random effects analysis, corrected for multiple comparisons using predefined regions of interest (ROIs) [small volume corrections (SVC)]. Amygdala and piriform cortex were selected as ROIs, based on their involvement in human olfactory processing7,8.
The mean t-statistics of "contrast of parameter estimate values" (COPEs) in each ROI were extracted for ME-EPI and 1E-EPI per participant. Two-tailed one-sample t-tests were performed for each condition.
Results
Piriform cortex activation from lemon odor stimulus was observed in ME-EPI but not in 1E-EPIConsistent with the previous findings9, activation with the lemon odor stimulus was observed bilaterally in the piriform cortex and in the left amygdala for ME-EPI (Figure 2). No activation survived correction for multiple comparisons for 1E-EPI.
Consistent with the voxel-based analysis, activation in the piriform cortex and amygdala reached significance in ROI-based analyses for the lemon odor stimulus (contrast: lemon > control) in ME-EPI, but not 1E-EPI (Figure 3).
Significantly higher BOLD signals were observed in the piriform cortex for ME-EPI
We extracted the mean t-statistics of COPEs from the piriform cortex (contrast: lemon > control) in each participant for both acquisition methods. ME-EPI showed significantly higher activation than 1E-EPI in the one-tailed paired t-test.
No significant activation from the benzaldehyde odor stimulus was observed in either acquisition method after multiple comparisons correction
Piriform cortex and amygdala activation were observed in ME-EPI, whereas only limited piriform cortex activation was observed in 1E-EPI (p<0.05, uncorrected, Figure 4). However, these effects did not survive multiple comparisons correction. Similarly, ROI analyses did not detect any significant activation for either region.
ME-EPI significantly reduces fMRI artifacts for olfactory-related tasks
Pronounced artifacts can be found in the ventricles and skull regions on the activation map for 1E-EPI, while with ME-EPI, most activations were contained within the cortices (Figure 4), consistent with ME-EPI’s demonstrated denoising performance6,10.
Discussion
We demonstrated that an optimized ME-EPI protocol can detect BOLD activation with fewer subjects and greater sensitivity than 1E-EPI. We believe this is due to two effects:1) Olfactory-related tasks can be confounded by respiratory and motion artifacts, which ME-EPI denoising rejects.
2) ME-EPI allows per-voxel combinations of echo times, addressing T2* variation within and across olfactory regions11.
Of note, not all stimuli produced significant activation in the piriform cortex, despite being perceptually intensity-matched, consistent with previous reports that activation of the piriform cortex varies from odorant to odorant1.
Taken together, these results strongly imply that our optimized ME-EPI provides distinct advantages over 1E-EPI for olfactory task-based fMRI.
Acknowledgements
This work was supported by the National Institutes of Health awards R01DC018075 and R01DC019405.
References
1. Deichmann, R., Gottfried, J. A., Hutton, C. & Turner, R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage 19, 430–441 (2003).
2. Glover, G. H. & Law, C. S. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine 46, 515–522 (2001).
3. Lynch, C. J. et al. Rapid Precision Functional Mapping of Individuals Using Multi-Echo fMRI. Cell Reports 33, 108540 (2020).
4. Gonzalez-Castillo, J. et al. Evaluation of multi-echo ICA denoising for task based fMRI studies: Block designs, rapid event-related designs, and cardiac-gated fMRI. NeuroImage 141, 452–468 (2016).
5. Posse, S. et al. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magnetic Resonance in Medicine 42, 87–97 (1999).
6. DuPre, E. et al. TE-dependent analysis of multi-echo fMRI with *tedana*. Journal of Open Source Software 6, 3669 (2021).
7. Zatorre, R. J., Jones-Gotman, M., Evans, A. C. & Meyer, E. Functional localization and lateralization of human olfactory cortex. Nature 360, 339–340 (1992).
8. Anderson, A. K. et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6, 196–202 (2003).
9. Gottfried, J. A., Deichmann, R., Winston, J. S. & Dolan, R. J. Functional Heterogeneity in Human Olfactory Cortex: An Event-Related Functional Magnetic Resonance Imaging Study. J. Neurosci. 22, 10819–10828 (2002).
10. Kundu, P., Inati, S. J., Evans, J. W., Luh, W.-M. & Bandettini, P. A. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage 60, 1759–1770 (2012).
11. Posse, S., Shen, Z., Kiselev, V. & Kemna, L. J. Single-shot T2* mapping with 3D compensation of local susceptibility gradients in multiple regions. NeuroImage 18, 390–400 (2003).
Figures

Figure 1. Experimental Paradigm for a Single Trial. A trial started with a fixation with an average duration of 4.5s, followed by a 1.5s countdown. A cue to sniff was then displayed and the odor was delivered for 2s. Discrimination tasks and intensity ratings were then performed. Each run contained 24 trials, equally divided among three odor stimuli. Each participant underwent 6 runs in total, with 3 runs of ME-EPI interleaved with 3 runs of single echo EPI, counterbalanced among 16 subjects.
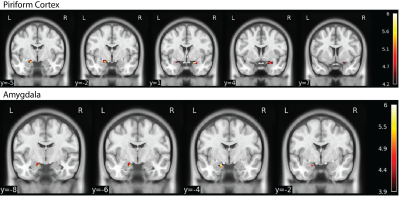
Figure 2. Main Effect of Lemon vs. Control for ME-EPI with SVC Correcting for Multiple Comparison. Consistent with previous studies, the main effect of lemon vs. control was observed in the bilateral piriform cortex and left amygdala for ME-EPI. However, with same the number of subjects, no effect was observed for 1E-EPI.
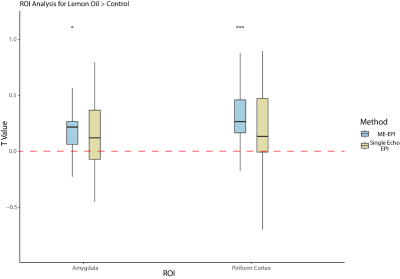
Figure 3. ROI Analysis for Lemon Oil > Control. The Piriform cortex and amygdala reached significance for ME-EPI (piriform cortex, p=0.0003; amygdala, p=0.0132) but not for 1E-EPI (piriform cortex, p=0.0504; amygdala, p=0.1586).
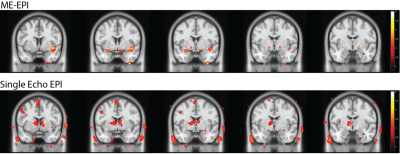
Figure 4. Main Effect of Benzaldehyde vs. Control (uncorrected, p<0.05). Without multiple comparisons correction, piriform cortex and amygdala activation were observed in ME-EPI, but only piriform cortex activation in 1E-EPI. However, the results did not survive multiple comparisons correction. Noticeably, ME-EPI has significantly fewer artifacts compared to the single echo EPI, such as in the ventricle and the skull regions.