0215
Design of a 64-Channel ex vivo Brain Rx Array Coil with field monitoring and temperature control for DWI at 3T1Institute of Medical Physics and Radiation Protection (IMPS), TH-Mittelhessen University of Applied Sciences, Giessen, Germany, 2Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3A. A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlstown, MA, United States, 4Harvard Medical School, Boston, MA, United States, 5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States, 6Center for Mind, Brain and Behavior (CMBB), Marburg, Germany
Synopsis
Keywords: RF Arrays & Systems, Brain
Long-durational diffusion weighted MRI scans with high gradient strength and high slew rate experiences in addition to the generally low signal-to-noise-ratio several problems, such as image artifacts due to eddy currents and the gradual increase of the sample temperature. Combining a high-density anatomically shaped receive coil with field monitoring and temperature control can overcome these limitations. Therefore, we designed and constructed a 64-channel whole human ex vivo brain Rx coil with integrated field monitoring and temperature control system. First SNR measurements confirm the receive capability with high SNR.Introduction
Diffusion Weighted Imaging (DWI) is a powerful method to probe the human brain structure. Compared to studies in living human subjects, ex vivo brain investigations provide various advantages. Close-fitting receive elements, high channel count, and ‘unlimited’ acquisition times boost the spatial and angular resolution and Signal-to-Noise-Ratio (SNR) and provide the possibility of validating in vivo results. However, studies have been showen that sample temperature increases during long DWI scans can distort measurement results [1]. Therefore, a temperature control system in the sample container is necessary to keep the brain temperature constant over long scan times (up to a couple of days).To increase the DWI quality, ultra-high gradient strength (500 mT/m) and slew rate (600 T/m/s) capabilities are being developed for human brain imaging [2]. However, the consequent increase in eddy currents and other system imperfections lead to artifacts such as distortion and ghosting [3]. A concurrent field monitoring system can mitigate this problem by measuring the spatiotemporal magnetic field dynamics during acquisition [3-5].
In this study, we designed and constructed a 64-channel ex vivo human brain array coil with a field monitoring and a temperature control system to be used with the new Connectome 2.0 head scanner [2,5]. Although, not yet in its final construction stage, preliminary results from the developed ex vivo Connectome 2.0 coil are presented.
Methods
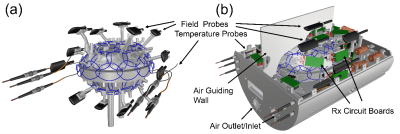
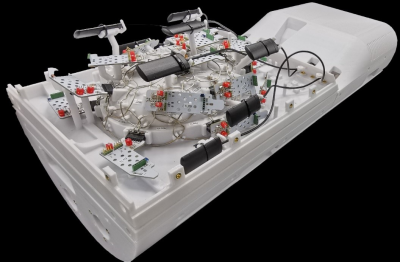
Rx coil: The coil former design is based on our previous ex vivo array coil [6] with slightly increased space to fit larger human brain samples (Fig. 1a). The increased number of 64 loop elements (average diameter = 50 mm) are decoupled geometrically (nearest neighbors) and by preamplifier decoupling. The adjacent elements of the top and bottom segment are also geometrically decoupled by an overlapping rim structure (Fig. 1a, Fig. 2). Due to the highly constrained radial space inside the Connectome 2.0 head-gradient coil, we densely packed and integrated all needed coil and field camera components into the coil hosing. Prior to construction, all details were 3D modelled to virtually optimize the component arrangement in the CAD program (Fig. 1b). Bench measurements with the assembled receive elements verified QU/QL-ratio, tuning, matching, decoupling, active detuning, and preamplifier decoupling.Image acquisition: SNR images and noise correlation were acquired on the 3T MAGNETOM Skyra Scanner (Siemens Healthcare GmbH, Erlangen, Germany) with an agar brain phantom using a proton density-weighted sequence and compared to a commercial 32ch head coil.
Field monitoring: The 16 field probes (Skope, Zurich, Switzerland) were distributed iteratively around the sample housing considering possible interactions with other coil parts. The probe positions were evaluated by computing the maximum phase error (MATLAB R2021a) [4,7,8] in comparison to the optimal probe configuration established for regular in vivo head coils [3].
Temperature control: The temperature control system consists of six fiber optic temperature probes (PRB-100-STM-MRI, OSENSA Innovations Corp. Burnaby, BC, Canada) around the sample and air flow through hoses into the sample container. Four probes are located within the coil housing to obtain the temperature inside the coil and two probes are placed at the sample volume to monitor brain peripheral temperature.
Results
Rx coil: Bench measurements showed an average preamplifier decoupling of 24.5 dB. Active detuning resulted in 37 dB isolation on average between the detuned and tuned state under loaded condition. The QU/QL-ratio was measured to be 180/77=2.34. The mean noise correlation (Fig. 3) was 11% compared to that of the comparison coil (22%). SNR measurements (Fig. 4) showed a substantially improved SNR in the representative acquired sagittal slice (3.5-fold and 1.2-fold SNR at the periphery and at the center, respectively), when compared to the standard 32-channel head coil.Field monitoring: The 16-channel field probe configuration with the lowest possible noise terms for the developed coil system was identified. The simulation of maximum phase error in comparison to the optimal in vivo probe configuration [3] shows, that noise conditioning is only slightly declined (Fig. 5).
Discussion
In long-duration diffusion-weighted MRI scans with ultra-high gradient strength and fast slew rate, several problems arise as signal distortions and ghosting caused by field dynamics and sample temperature increase [1,3]. Our 64-channal ex vivo brain coil addresses these limitations, by incorporating a concurrent field monitoring system and a forced air temperature control system. The coil provides high SNR mainly due to the close-fitting anatomical shape and the high channel count.Finding the optimal field camera configuration around the coil former, with loop elements, preamplifiers, cable traps, mechanical structures and field probes sharing the same space, was a challenging undertaking. Iterative re-arrangement under strict simulation guidance resulted in the final conditioning of the field probe noise, which was only slightly increased compared to a standard in vivo head coil configuration. Our simulations suggest that the configuration of the camera system enables the capturing of up to 3rd order field dynamics.
Conclusion
The combination of a 64-channel ex vivo array for whole human brain specimens with concurrent magnetic field monitoring, and the ability to control the sample temperate, will provide increased stability allowing acquisitions of high resolution and high SNR diffusion-weighted images with reduced image artifacts.Acknowledgements
No acknowledgement found.References
[1] D’Arceuil, Helen E., Susan Westmoreland, and Alex J. de Crespigny. "An approach to high resolution diffusion tensor imaging in fixed primate brain." Neuroimage 35.2 (2007): 553-565.
[2] Huang, Susie Y., et al. "Connectome 2.0: Developing the next-generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso-and macro-connectome." NeuroImage (2021): 118530.
[3] Wilm, Bertram J., et al. "Diffusion MRI with concurrent magnetic field monitoring." Magnetic resonance in medicine 74.4 (2015): 925-933.
[4] Barmet, Christoph, Nicola De Zanche, and Klaas P. Pruessmann. "Spatiotemporal magnetic field monitoring for MR." Magnetic Resonance in Medicine 60.1 (2008): 187-197.
[5] Ramos-Llorden, Gabriel et al. “Distortion-and ghosting-free high b-value ex vivo human brain diffusion MRI achieved with spatiotemporal field monitoring.” Proceedings of the 2022 Annual Meeting of ISMRM, London, UK. 232.
[6] Scholz, Alina, et al. "A 48-Channel Receive Array Coil for Mesoscopic Diffusion-Weighted MRI of ex vivo Human Brain on the 3 T Connectome Scanner." NeuroImage (2021): 118256.
[7] Barmet, Christoph, et al. "Concurrent higher-order field monitoring for routine head MRI: an integrated heteronuclear setup." Proceedings of the 18th Annual Meeting of ISMRM, Stockholm, Sweden. Vol. 216. 2010.
[8] Mahmutovic, Mirsad, et al. “A 64-Channel Brain Array Coil with an Integrated 16-Channel Field Monitoring System for 3T MRI”. Annual Meeting of Intl Soc Magn Reson Med, Virtual Meeting (2021) #0623.
Figures