0207
Experimental Assessment of the Effects of Subject Motion on Local SAR and pTx Pulse Performance at 7T1Imaging Centre of Excellence, University of Glasgow, Glasgow, Scotland, 2Siemens Healthcare Ltd., Frimley, United Kingdom, 3NHS GGC MRI Physics, NHS Greater Glasgow & Clyde, Glasgow, Scotland, 4MR CoilTech Ltd., Glasgow, Scotland, 5Magnetic Detection & Imaging Group, University of Twente, TechMed Centre, Netherlands
Synopsis
Keywords: Parallel Transmit & Multiband, Safety, RF Pulse Design, RF Coils
Previous simulation studies of motion in parallel transmission (pTx) have identified large increases in specific absorption rate (SAR) and pulse performance error. This abstract extends this by evaluating the effects of motion on RF shimming and dynamic pTx in vivo at 7T. The T1w image of a healthy volunteer was segmented to create a body model for electromagnetic simulation in a custom pTx coil. The model was simulated at six head positions matched to experimental measurements. Motion had a detrimental effect on pTx pulse performance and also caused local SAR changes, though not as severe as seen previously in simulation.Introduction
With increasing prevalence of ultra-high field (UHF) MRI, parallel transmission (pTx) is gaining popularity as an important tool for mitigating RF field (B1+) inhomogeneity. Recently, fully-integrated or calibration-free methods such as Universal Pulses (UPs)1, Fast Online CUStomized (FOCUS) pulses2, and Direct Signal Control (DSC)3 have improved the cumbersome pTx workflow and broadened usage.The effects of subject motion on pTx performance have recently been investigated in comprehensive simulation studies.4,5,6,7,8 These works reported substantial degradation of pTx pulse fidelity with increases in normalized root mean squared error (NRMSE) of up to ~35%7 and increases in local specific absorption rate (SAR) of up to ~300%.8 Currently, such SAR variations need to be accounted for in the SAR model, for example by assuming the worst-case eigenvalue or as virtual observation points (VOPs)9 in conjunction with further safety factors. Despite improvements in B1+ estimation via deep learning8, updating the pTx pulse design to address motion-induced degradation remains an open challenge.
So far, the UHF community has relied heavily on generic body models and relatively wide motion ranges to assess local SAR variability due to head position variations. Alternatively, introducing improved segmentation schemes tolerant to B1+ image shading can provide accurate subject-specific SAR estimates in the head10. Using a subject-specific electromagnetic (EM) body model, this abstract presents preliminary work that improves upon previous simulation-only studies by assessing pTx performance with actual subject motion for the first time in vivo at 7T.
Methods
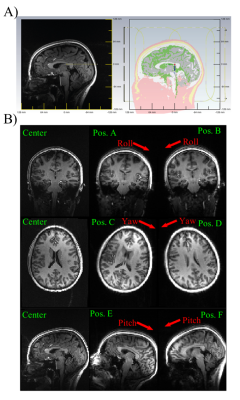
Following informed consent, a healthy volunteer was scanned in a 7T MRI system (MAGNETOM Terra, Siemens Healthineers, Germany) using a custom 8Tx/32Rx head coil11 and with the subject positioned in the center of the coil. First, a T1w 3D MPRAGE scan (TR/TE/TI=2500/2.2/1050ms, FA/GRAPPA/FOV/Res=5॰/R=2/250x250x192mm3/1mm3, TA=5:45min) was used to create an individualized EM body model of the head following the method in Ref. 7. Additionally, single-channel B1+ maps were collected with a presaturated TurboFLASH method12 (TR/TE =7340/1.6ms, FA/FOV/Res/Slice Thickness=5॰/250x250mm2/5mm2/4mm, TA=1:31min).The subject was then asked to move along six prescribed motion directions, i.e. along the roll, yaw, and pitch rotation axes (shown in Figure 1b), to the maximum capacity allowed by the tight-fitting receive helmet. In each position, a shorter T1w-MPRAGE scan was acquired (TA=2:00min, Res=2mm3, all other parameters constant) along with single-channel B1+ maps. All anatomical scans were performed in CP mode.
Rigid registration of T1w images was performed in FSL13 using six degrees-of-freedom to identify rotations and translations with respect to the reference data acquired in center position. These transformations were then imposed on the subject-specific EM body model in CST Microwave Studio Suite (Dassault Systems, France) to simulate B1+ and Q-matrices14 for all head positions.
Experimental B1+ maps were used to assess motion effects on pTx pulse performance in terms of NRMSE. First, slice-specific RF shims for all transverse slices were optimized for the center position and then evaluated on all head positions, along with CP excitation and a position-specific RF shims set. Next, non-selective excitation and inversion pulses were designed for the center position with the FOCUS method2 and again evaluated for all head positions.
Finally, local SAR effects were evaluated in all head positions for both the RF shims and non-selective pTx pulses described above.
Results
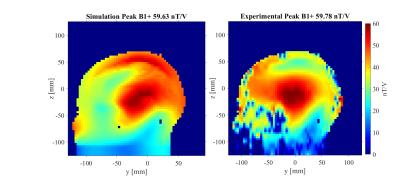
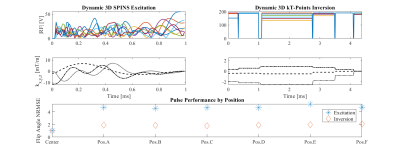
Figure 2 compares the simulated and measured B1+ maps for the subject positioned in the center of the coil.Figure 3 shows NRMSE performance of slice-specific shimming in the center and 6 offset positions, while Figure 4 shows the corresponding results for the 3D excitation and inversion pulses.
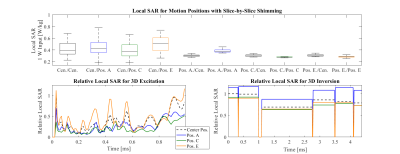
Figure 5 shows the simulated subject-specific local SAR for the center position and offset head positions A, C, and E, for the same slice-specific shims and the 3D dynamic FOCUS pulses evaluated in Figures 3 and 4.
Discussion & Conclusion
This experimental analysis of motion effects on pTx with a subject-specific EM body model showed similar results to previous simulations with substantial degradation of pTx pulse performance7. For slice-by-slice shimming, each offset head position produced NRMSE values higher than CP excitation at center position. Meanwhile, the NRMSE in corresponding dynamic pTx pulses increased by 2-5x (Fig. 4). With the continued effort in pTx pulse design, this detriment is an important consideration for all pTx users. With the increased usage of pTx and likelihood of less compliant subjects and patient exams, these results warrant further simulation and in vivo studies.Changes in SAR were seen for varying head positions (Fig. 5). Importantly, the local SAR distribution decreased when slice-specific RF shims were re-optimized. This is likely due to the reduced activation of elements closer to the head, compared to using center-optimized settings. For dynamic pTx, different head positions exhibited different local SAR across time with the worst position and time point creating 16% higher SAR for excitation and 17% for inversion. Encouragingly, this is well-below the SAR increases reported previously8, which might reflect the more realistic motion ranges considered here.
One limitation is the match between the simulated and measured B1+ maps, yet future work will refine this agreement with additional segmentation model training. Furthermore, the computation time required for EM simulations, currently amounting to several 10s of hours, is a substantial bottleneck in evaluating additional body models and head positions.
Acknowledgements
*SNW and PM contributed equally to this work.We would also like to thank the radiographers from the Imaging Centre of Excellence, University of Glasgow.References
1. Gras V, Vignaud A, Amadon A, et al. Universal pulses: a new concept for calibration-free parallel transmission. Mag. Reson. Med. Feb. 2017. Vol 77:2, p. 635-643.
2. Herrler J, Liebig P, Gumbrecht R, et al. Fast online-customized (FOCUS) parallel transmission pulses: a combination of universal pulses and individual optimization. Mag. Reson. Med. Jun. 2021. Vol 85:6, p. 3140-3153.
3. Tomi-Tricot R, Sedlacik J, Endres J, et al. Fully integrated Scanner implementation of direct signal Control for 2D T2-weighted TSE at ultra-high Field. Proc Int Soc Mag Reson. May 2021. p. 0621.
4. de Greef M, Ipek O, Raaijmakers A, et al. Specific absorption rate intersubject variability in 7T parallel transmit MRI of the head. Mag. Reson. Med. May. 2020. Vol 69:5, p.1476-1485.
5. Kopanoglu E, Deniz C, Erturk MA, and Wise RG.Specific absorption rate implications of within-scan patient head motion for ultra-high field MRI. Mag. Reson. Med. Nov. 2020. Med. Vol 84:5, p. 2724-2738.
6. Ajanovic A, Brackenier Y, Tomi-Tricot R et al. Motion and pose variability of SAR estimation with parallel transmission at 7T. Proc Int Soc Mag Reson. May 2021. p. 2487.
7. Plumley A, Watkins L, Treder M, et al. Rigid motion-resolved B1+ prediction using deep learning for real-time parallel-transmission pulse design. Mag. Reson. Med. Dec. 2021. Vol 87, p. 2254–2270.
8. Plumley A, Goodwin N, and Kopanoglu E. Inter-subject differences in SAR sensitivity to motion with parallel-transmit at 7T. Proc Int Soc Mag Reson. May 2022. p. 0457.
9. Eichfelder G and Gerbhardt G. Local specific absorption rate control for parallel transmission by virtual observation points. Mag. Reson. Med. Nov. 2011. Vol 66:5, p. 1468-1476.
10. Brink WM, Yousefi S, Bhatnagar P, et al. Personalized local SAR prediction for parallel transmit neuroimaging at 7T from a single T1-weighted dataset. Mag. Reson. Med. Mar. 2022. Vol 8, p. 464-475.
11. Williams SN, Allwood-Spiers S, McElhinney P, et al. A nested eight-channel transmit array with open-face concept for human brain imaging at 7 tesla. Front Phys. Jul 2021. Vol 9.
12. Fautz HP, Vogel M, Gross P, et al. B1 mapping of coil arrays for parallel transmission. Proc Int Soc Mag Reson. May 2008. p. 1247.
13. Jenkinson M, Bannister P, Brady, JM and Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. Oct. 2002. Vol 17:2, p. 825-841.
14. Graesslin I, Homann H, Biederer S, et al. A specific absorption rate prediction concept for parallel transmission MR. Mag. Reson. Med. Jan. 2012. Vol 68, p. 1664-1674.
Figures