0193
Automated breast tumor segmentation in DWI using multi-modality image registration: a feasibility study using multi-center data1Radiology, University of California, San Francisco, San Francisco, CA, United States, 2Epidemiology & Biostatistics, University of California, San Francisco, San Francisco, CA, United States, 3Radiology, University of Minnesota, Minneapolis, MN, United States, 4Radiology, University of Washington, Seattle, WA, United States
Synopsis
Keywords: Breast, Cancer
Diffusion-weighted imaging (DWI) in MRI plays an important role in diagnostic applications. Automated tumor segmentation is an important yet challenging step for quantitative breast imaging analysis. While methods have been developed for dynamic contrast enhanced (DCE) MRI, automatic segmentation in breast DWI-MRI is still underdeveloped. The purpose of this study is to develop methods to transfer functional tumor volume (FTV) analysis ROI from DCE-MRI image space to DWI-MRI image space using image registration and extract ADC statistics in regions corresponding to those of DCE segmentations for prediction of pathologic treatment response (pCR).Background
Clinical MR breast imaging is often performed using dynamic contrast-enhanced imaging (DCE), currently a routine clinical exam with existing standards for image acquisition and interpretation (1). However, diffusion-weighted imaging (DWI), an MR technique that tracks the random motion of water molecules, has advantages for measuring tumor responses to treatment. Apparent diffusion coefficient (ADC) is a quantitative measure derived from two or more DWI images with different b-values, that has been shown to correlate with tumor response in the neoadjuvant chemotherapy (NAC) setting (2–4).Although manual segmentation for delineating the region-of-interest (ROI) in an ADC map conventionally considered as gold-standard has previously been reported (5,6), it is time consuming, error-prone, and subject to inter-user inconsistency. There are few fully automated methods for tumor segmentation on breast DWI (7,8), and to our knowledge, no fully automated tumor segmentation has been applied to breast DWI MRI. Fortunately, DWI and DCE image sequences are often acquired in the same MRI exam, therefore we can obtain DWI segmentations by applying segmentations from DCE images to corresponding DWI images after co-registration.
The purpose of this retrospective study was to develop an automated tumor segmentation approach for breast DWI by co-registering the DWI and DCE image series to allow application of the DCE segmentations on the DWI ADC maps. Using the multi-center DWI data from ACRIN 6698, we compared ADC values derived from manually delineated and transformed ROIs. Finally, we evaluated the utility of ADC values derived using transformed and manual ROIs for predicting pathologic complete response (pCR).
Methods
In the prospective multicenter ACRIN 6698 trial - a substudy of ISPY2 (9,10), 272 women with breast cancer were enrolled and randomized to treatment with 12 weekly doses of paclitaxel. Each woman underwent breast MRI including DCE and DWI before treatment (T0), at early treatment (T1), mid-treatment (T2), and after treatment (T3). Functional tumor volume (FTV) ROIs were obtained from DCE images using a semi-automatic standardized method (11).Tumor regions on DWI were manually delineated by trained researchers at University of California, San Francisco, who were blinded to pathologic outcomes. Primary analysis of ACRIN 6698 found ADC to be statistically significant associated with pCR at T2 but not at T1 (12).For this study, MRI exams acquired at T0 and T1 were analyzed. Percentage change in tumor ADC compared to T0 (ΔADC) was calculated. ADC mean, standard deviation, skewness and kurtosis were extracted from both manual and transformed ROIs. Bland-Altman plots were used to assess difference between manual and transformed ROIs. Performance for predicting pCR was assessed with the area under the receiver operating characteristic curve (ROC-AUC) of logistic regression for ΔADC from both manual and transformed ROIs. ROC-AUCs were compared by bootstrapping with 2,000 replicates using a two-sided test. Registration was implemented in Elastix (13) in two steps. First, rigid transformations were performed successively to align and match the features of the fixed (B0 DWI) and moving (Pre-contrast DCE) images. Second, a B-spline transformation with bending energy regularization penalty was performed to elastically refine the alignment. The combined transformation was then applied to the DCE ROI to generate a transformed ROI in the DWI image. The segmentation performance was measured using the Dice Coefficient (DC) for agreement between manual segmentation and transformed ROIs.Results
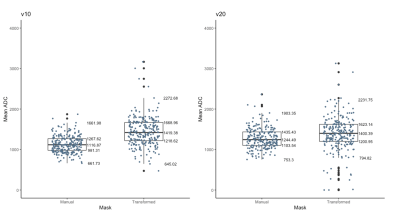
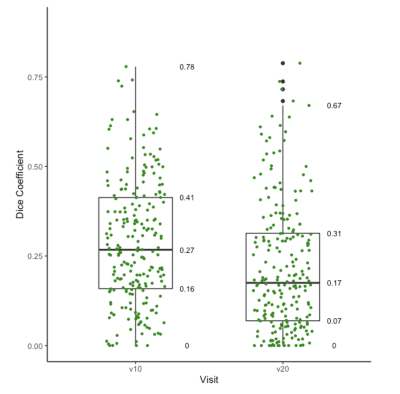
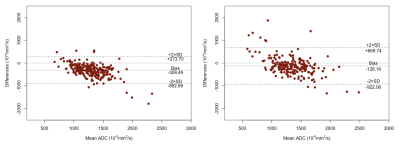
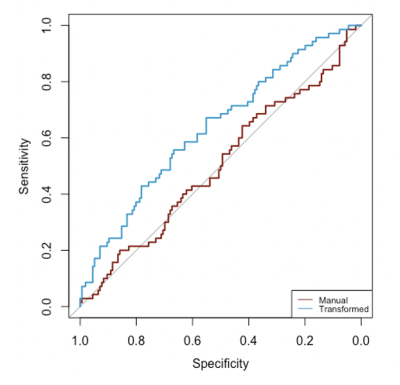
The final analysis included 226 patients with evaluable imaging data, manual ROI, and treatment outcome, with a mean age of 48 years ± 10 (standard deviation) and 70 (33%) of 226 patients experienced pCR. Median ADC from the manual ROI approach at T0 and T1 were 1116 [981-1267] x 10-6 mm2/s and 1244 [1103-1435] x 10-6 mm2/s, respectively. Median ADC based on the transformed ROI at T0 and T1 were 1419 [1218-1669] x 10-6 mm2/s and 1400 [1200-1623] x 10-6 mm2/s, respectively (Figure 1). Median Dice Coefficient of T0 is 0.27 [0.16-0.41], whereas for T1 it was 0.17 [0.07-0.31]. The boxplots of Dice Coefficient are shown in Figure 2. The Bland-Altman plots comparing ADCs derived from manual and transformed ROIs at T0 and T1 are shown in Figure 3. ΔADC did not appear to be predictive of pCR at T1 (AUC = 0.50 [95% CI: 0.42, 0.59]) based on the manual ROI but did indicate predictive value of pCR at T1 (AUC = 0.64 [0.56, 0.71]) based on the transformed ROI (Figure 4). The p-value testing for a difference between the two AUCs was 0.004.Discussion
Tumor segmentation is a challenging task. We applied a registration-based method to transform breast tumor segmentation from pre-contrast DCE MRI to B0 DWI. The two segmentation processes result in very different pixel selection for inclusion in the ROI, as highlighted by the low Dice coefficients. The predictive performance of the change in ADC was improved when using transformed ROIs, leading to statistically significant prediction of pCR from ADC at early treatment that was not found in the primary analysis of ACRIN 6698. Therefore, these results using a registration-based segmentation strategy are highly encouraging.Conclusion
In conclusion, we investigated tumor segmentation on breast DWI using image registration from pre-contrast DCE MRI data. This work provides a practical approach for automated breast tumor segmentation and initial results indicate improved predictive value for pCR over manual segmentation.Acknowledgements
Acknowledgements: National Institutes of Health Grants: NIH/NCI R01 CA132870, U01 CA225427, P01 CA210961, R01 CA255442, R01 CA190299.References
1. Partridge SC, McDonald ES. Diffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applications. Magn Reson Imaging Clin N Am [Internet]. 2013/06/10. 2013 Aug;21(3):601–24. Available from: https://pubmed.ncbi.nlm.nih.gov/23928248
2. Jensen LR, Garzon B, Heldahl MG, Bathen TF, Lundgren S, Gribbestad IS. Diffusion-weighted and dynamic contrast-enhanced MRI in evaluation of early treatment effects during neoadjuvant chemotherapy in breast cancer patients. J Magn Reson Imaging. 2011 Nov;34(5):1099–109.
3. Galbán CJ, Ma B, Malyarenko D, Pickles MD, Heist K, Henry NL, et al. Multi-Site Clinical Evaluation of DW-MRI as a Treatment Response Metric for Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. PLoS One [Internet]. 2015;10(3):1–19. Available from: https://doi.org/10.1371/journal.pone.0122151
4. Yuan L, Li JJ, Li CQ, Yan CG, Cheng ZL, Wu YK, et al. Diffusion-weighted MR imaging of locally advanced breast carcinoma: the optimal time window of predicting the early response to neoadjuvant chemotherapy. Cancer Imaging. 2018 Oct;18(1):38.
5. Belli P, Costantini M, Ierardi C, Bufi E, Amato D, Mule’ A, et al. Diffusion-weighted imaging in evaluating the response to neoadjuvant breast cancer treatment. Breast J. 2011;17(6):610–9.
6. Gity M, Moradi B, Arami R, Arabkheradmand A, Kazemi MA. Two Different Methods of Region-of-Interest Placement for Differentiation of Benign and Malignant Breast Lesions by Apparent Diffusion Coefficient Value. Asian Pac J Cancer Prev. 2018 Oct;19(10):2765–70.
7. Benjelloun M, Adoui ME, Larhmam MA, Mahmoudi SA. Automated Breast Tumor Segmentation in DCE-MRI Using Deep Learning. In: 2018 4th International Conference on Cloud Computing Technologies and Applications (Cloudtech). 2018. p. 1–6.
8. Carvalho ED, Silva RRV, Mathew MJ, Araujo FHD, Filho AODC. Tumor Segmentation in Breast DCE- MRI Slice Using Deep Learning Methods. In: 2021 IEEE Symposium on Computers and Communications (ISCC). 2021. p. 1–6.
9. Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, et al. The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. J Digit Imaging. 2013;26(6):1045–57.
10. Newitt DC, Partridge SC, Zhang Z, Gibbs J, Chenevert T, Rosen M, et al. ACRIN 6698/I-SPY2 Breast DWI [Data set]. The Cancer Imaging Archive. 2021;
11. Newitt DC, Aliu SO, Witcomb N, Sela G, Kornak J, Esserman L, et al. Real-time measurement of functional tumor volume by MRI to assess treatment response in breast cancer neoadjuvant clinical trials: validation of the aegis SER software platform. Transl Oncol. 2014;7(1):94–100.
12. Partridge SC, Zhang Z, Newitt DC, Gibbs JE, Chenevert TL, Rosen MA, et al. Diffusion-weighted MRI Findings Predict Pathologic Response in Neoadjuvant Treatment of Breast Cancer: The ACRIN 6698 Multicenter Trial. Radiology. 2018 Dec;289(3):618–27.
13. Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2009;29(1):196–205.
Figures