0183
Free-breathing, Whole-heart, High-dynamic-range Quantitative Susceptibility Mapping (HDR-QSM) for Imaging Hemorrhagic Infarctions
Yuheng Huang1,2, Xingmin Guan1, Xinheng Zhang1,2, Liqi(Richard) Tang 1, Xiaoming Bi3, Fei Han3, HsuLei Lee4, Hui Han4, Anthony Christodoulou4, Debiao Li4, Rohan Dharmakumar1, and Hsin-Jung Yang4
1krannert cardiovascular research center, Indiana University school of medicine, Indianapolis, IN, United States, 2Bioengineering, UCLA, LA, CA, United States, 3Siemens Healthineers, Malvern, PA, United States, 4Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States
1krannert cardiovascular research center, Indiana University school of medicine, Indianapolis, IN, United States, 2Bioengineering, UCLA, LA, CA, United States, 3Siemens Healthineers, Malvern, PA, United States, 4Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Keywords: Myocardium, Cardiovascular, hemorrhagic reperfusion injury myocardial infarction
Accurately detecting and quantifying intramyocardial hemorrhage(IMH) is critical for patient management. QSM has evolved into the standard method for iron imaging. However, obtaining cardiac QSM for IMH evaluation is difficult due to well-known technical challenges. Here, we developed a motion-robust 3D multi-echo GRE technique and combined them with a high-dynamic-range QSM algorithm to derive reliable QSM maps in IMH hearts. We tested and validated it in phantom, ex-vivo, and in-vivo IMH hearts in animal models. We demonstrated that the proposed method could accurately detect IMH and reliably quantify iron concentration with a free-breathing scan under 6 minutes.Introduction
Timely restoration of blood flow to the ischemic myocardium can reduce infarct size and improve outcomes for patients undergoing acute myocardial infarction (MI). However, opening up obstructed coronary arteries under acute MI is accompanied by the risk of inducing hemorrhagic reperfusion injury and further damage to the heart. Recent studies showed that accurate detection and characterization of intramyocardial hemorrhage (IMH) following re-perfused MI are important for the advancement of understanding and therapies to limit the detrimental effects of IMH in the heart. QSM has evolved into the standard method for iron imaging in the brain. However, its application in the heart has been limited by major technical shortcomings. Confounders, like the involuntary cardiac and respiratory motion1, the large B0 inhomogeneity at the heart-lung interfaces, and the streaking artifacts in cases of IMH with high iron concentration, make cardiac QSM challenging. In this study, we developed a free-breathing, motion-mitigated whole-heart QSM technique with a High Dynamic Range phase reconstruction algorithm (HDR-QSM). HDR-QSM overcomes key confounders for imaging IMH based on focal iron deposition and provides highly reliable QSM measurements across a wide range of field disturbances and iron concentrations.Methods
QSM images were acquired in phantoms with a range of iron concentrations(0.18-1.8 mM) and animal models with IMH. Under institutional approval, canines subjected to hemorrhagic MIs (n=10) were studied seven days post-MI. Animals were scanned in a clinical 3T scanner(Siemens). In the in-vivo scans, a 3D, non-ECG gated, free-breathing 8-echo GRE (mGRE) sequence was prescribed to cover the whole LV and reconstructed using an LRT framework (TE1/ΔTE = 1.42/2.01ms, Slice number = 12, voxel size 1.6×1.6×6 mm3)2. For validation purposes, post-euthanization ex-vivo hearts (n=5) were scanned with similar TEs to acquire images without interference from motion and air-tissue interfaces. The same imaging parameters were adopted for the phantom studies. An HDR-QSM reconstruction pipeline was developed to eliminate common confounders in imaging IMH (Fig.1). Briefly, a previously proposed guided phase unwrapping approach was adopted3 and combined with the SPURS algorithm for chemical shift correction4. Following, the unwrapped phase maps were combined using an SNR-weighted nonlinear least squares fitting algorithm4 (With cut-off: SNR = 8) for a high-fidelity phase map. Then the cardiac phase maps were processed with a two-step QSM algorithm (λ1 = 1,000, λ2 = 5,000)5 to minimize streaking artifacts from the high iron concentration in IMH lesions. Results from the proposed HDR-QSM were compared with conventional iron-sensitive images (R2*(1/T2*) maps and standard QSM(MEDI QSM, λ = 1,000)6 derived from the same acquisition.Results
Results from the phantom study are shown in Fig.2. Panel A showed representative T2* weighted images, R2* maps, and HDR-QSM images. The relationship between the HDR-QSM susceptibility measurements, R2* values, and iron concentration are compared in panel B. strong linear relationships(R-square: 0.998 and 0.985) are presented between the susceptibility, R2*, and iron concentration. The high linearity shows the potential for accurate iron quantification using HDR-QSM. Fig.3 shows representative in-vivo images from an IMH dog. Iron-sensitive images and the corresponding LGE images are presented for three slices in the heart. In the conventional iron-sensitive images, strong off-resonance artifacts were present at the heart-lung interfaces (red boxes) but were absent in the HDR-QSM maps. In addition, HDR-QSM corrected the strong streaking artifacts around the IMH zone in the standard QSM images (green boxes) and demonstrated a more homogeneous susceptibility measure in the lesions. Fig. 4 shows representative ex-vivo images from the same heart. In the IMH zone, similar trends to the in-vivo images are presented. Without the off-resonance effect from the heart-lung interfaces, apparent signal elevations from focal iron deposition are presented in the R2* maps. Good spatial correspondence of the regions is also shown in the QSM images. In addition, HDR-QSM showed reduced streaking artifacts from the hemorrhagic core and presented a tighter correspondence to the R2* maps(green boxes). In Fig.5, ROC analysis was performed on the in-vivo images using ex-vivo T2* maps (Mean-2×SD) as the ground truth. The AUC of HDR-QSM was significantly higher than other approaches, which reflects the successful elimination of the susceptibility-induced error in the conventional modalities (AUC: R2*=0.83; standard QSM=0.69; HDR-QSM=0.94).Discussion
In this study, we developed a free-breathing cardiac QSM technique to quantify iron deposition in hemorrhagic myocardial infarction. Because IMH patients often suffer from compromised breath-holding capability and irregular heart motion, a non-ECG gated and free-breathing imaging technique enhanced the potential success rate in acquiring reliable images for IMH assessment. The proposed algorithm successfully mitigated the disruptive off-resonance artifacts at the heart-lung interfaces and the streaking artifacts in the hemorrhagic core from the standard QSM algorithm. This boosted the sensitivity and specificity of IMH detection compared to the standard quantitative approaches (Standard QSM and R2* maps) and showed the ability for reliable and accurate iron quantification in infarcted hearts. The next step is to test the developed technique in IMH patients in a clinical setting.Conclusions
We have developed an HDR-QSM technique that mitigates the persistent susceptibility-induced imaging artifacts in iron-sensitive CMR. HDR-QSM opens the door for robust iron quantification in the heart. It shines a light on precision care for IMH patients and can facilitate the development of advanced therapy development for hemorrhagic hearts.Acknowledgements
This work was supported by NIH 1R01HL148788 and NIH 5R01HL147133
References
[1]. Wen Y, Nguyen TD, Liu Z, et al. Cardiac quantitative susceptibility mapping (QSM) for heart chamber oxygenation. Magn Reson Med. 2018;79(3):1545-1552. doi:10.1002/mrm.26808 [2]. Guan X, Yang HJ, Hu Z, Wang N, Christodoulou A, Sharif B, Li D, Dharmakumar R. Free-breathing, Fully-Ungated, 3D Cardiac T2* MR Mapping using a Low-Rank Tensor Framework, 2022 Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting(2022) [3]. Huang Y, Zhang X, Fradad S, Meng L, Yoosefian G, Azab L, Li X, Kwan A, Dharmakumar R, Han H, and Yang HJ. Accurate mUlti-echo phase image wiTh uneven echO spacing and Ultra-High Dynamic Range (AUTO-HDR), Proceedings of the 28th Scientific Annual Meeting of the ISMRM Virtual Meeting (2020) [4]. Dong J, Liu T, Chen F, et al. Simultaneous phase unwrapping and removal of chemical shift (SPURS) using graph cuts: application in quantitative susceptibility mapping. IEEE Trans Med Imaging. 2015;34(2):531-540. doi:10.1109/TMI.2014.2361764 [5]. Wei H, Dibb R, Zhou Y, et al. Streaking artifact reduction for quantitative susceptibility mapping of sources with large dynamic range. NMR Biomed. 2015;28(10):1294-1303. doi:10.1002/nbm.3383 [6]. Liu T, Xu W, Spincemaille P, Avestimehr AS, Wang Y. Accuracy of the morphology enabled dipole inversion (MEDI) algorithm for quantitative susceptibility mapping in MRI. IEEE Trans Med Imaging. 2012;31(3):816-824. doi:10.1109/TMI.2011.2182523Figures
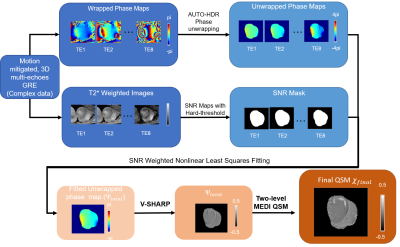
Reconstruction flow chart
for HDR-QSM: First,
phase maps at each echo were unwrapped using the AUTO-HDR unwrapping algorithm3. In parallel, SNR maps were
derived from the corresponding magnitude images with hard thresholding(SNR =
8). Then, the total field map was obtained using an SNR-based weighted
non-linear least squares fitting algorithm4. The
QSM image was derived using a PDF background removal algorithm with a two-level
MEDI approach4,5.
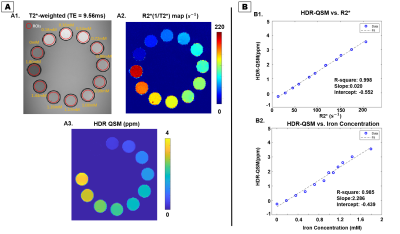
Iron-sensitive images from the Iron phantom study: In panel A, T2* weighted
images(A1), R2*(1/T2*)(A2), and HDR-QSM(A3) are shown. The relationships
between the proposed QSM measurements, R2*, and iron concentration are presented
in panel B. The proposed method demonstrate good linear correlation with R2*(B1,
R-square: 0.998), and with Iron concentration(B2, R-square: 0.985).
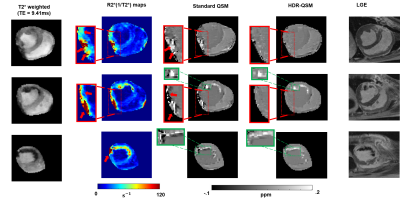
Iron-sensitive images reconstructed from the same imaging multi-echo GRE data: Representative images of a dog with IMH at basal, mid, and apical slices are presented. All the images except for LGE images are obtained from the same multi-echo GRE data. In R2* and standard QSM(MEDI) images, regions affected by off-resonance artifacts at the lateral wall are highlighted and zoomed-in (red boxes). Regions affected by streaking artifacts at the periphery of the hemorrhagic lesion are enlarged and illustrated in green boxes. Corresponding LGE images are also shown for reference.
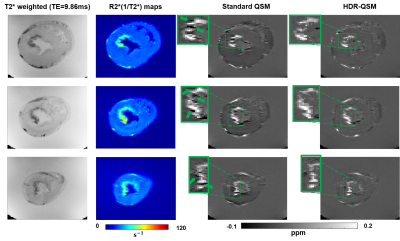
Iron-sensitive images
from post-euthanization
ex-vivo heart: Representative images from an ex-vivo dog heart with IMH at basal, mid,
and apical slices are presented. In T2* weighted images, R2*(1/T2*) and
standard QSM(MEDI) images, and proposed HDR-QSM images, hemorrhagic regions were
picked up by all types of images. However, streaking artifacts exists in the standard QSM approach. Regions affected by streaking artifacts at the
periphery of the hemorrhagic lesions are enlarged and illustrated in green boxes.
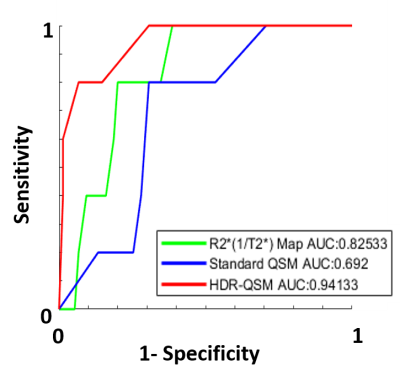
ROC analysis for IMH detection
using HDR-QSM, standard QSM, and R2* maps: ROC analysis shows that HDR-QSM
provides significantly improved accuracy in IMH detection compared to
conventional iron-sensitive CMR images. The boosted AUC from the HDR-QSM
reflects the successful elimination of notorious off-resonance-induced imaging artifacts
in the heart and provides a robust way to quantify iron lesions in IMH.
DOI: https://doi.org/10.58530/2023/0183