0179
Free-Breathing Simultaneous Native Myocardial T1, T2, and T1ρ Mapping with Cartesian Acquisition and Dictionary Matching1School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 2Shanghai Clinical Research and Trial Center, Shanghai, China, 3Department of Cardiovascular Medicine, Ruijin Hospital Lu Wan Branch, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 4School of Medical Technology, Beijing Institute of Technology, Beijing, China, 5iHuman Institute, School of Life Science and Technology, ShanghaiTech University, Shanghai, China
Synopsis
Keywords: Myocardium, Tissue Characterization
T1 and T2 are well-recognized parameters for detecting various cardiomyopathies, and T1ρ is an endogenous contrast for myocardial fibrosis. Multi-parametric cardiac MRI can provide co-registered parameter maps for comprehensive diagnosis and account for the interparameter dependency for more accurate quantification. Therefore, we propose a free-breathing simultaneous T1, T2, and T1ρ mapping technique with single-shot Cartesian acquisition and dictionary matching for parameter quantification. The phantoms results indicated the good accuracy and insensitiveness to heart rates of the proposed technique. Using prospective through-plane and retrospective in-plane motion correction, the proposed method generated similar in vivo mapping quality to breath-hold mapping methods.
Introduction
Cardiac T1 and T2 mapping have been shown to provide unique insights into cardiomyopathic tissue changes. T1 can be used to detect amyloid, inflammation and fat infiltration, while T2 is sensitive to edema 1. T1ρ is sensitive to the slow motion of macromolecules within the lattice, and has shown the potential for diagnosing focal and diffuse myocardial fibrosis 2,3. Simultaneous estimation of multiple parameters can provide complementary information for diagnosis and prognosis 4. A cardiac MR fingerprinting technique has been proposed for simultaneous T1, T2 and T1ρ mapping, which however requires complex reconstruction and a breath-hold of ~16s 5. Another relevant work is the Cartesian dictionary-based joint T1 and T2 mapping technique 6 which is also performed under breath-hold. In this study, we aim to propose a multi-parametric mapping sequence that can provide co-registered T1, T2, and T1ρ maps, where diaphragmatic navigator 7,8 was adopted to correct through-plane respiratory motion and in-plane motion was corrected retrospectively. Quantitative parameters were obtained using dictionary matching to account for subjective heart rates and B1 inhomogeneities. Phantom and in vivo imaging experiments were performed to validate the proposed technique.Methods
The framework of the proposed technique is shown in Fig. 1. The sequence consists of 16 single-shot acquisitions with a bSSFP readout and Cartesian sampling. Inversion recovery (IR), T2 preparation (T2-prep) and T1ρ preparation (T1ρ-prep) pulses are introduced to induce varying T1, T2, and T1ρ contrasts. The T1ρ-prep module consists of an adiabatic half-passage (AHP) tip-down pulse, a continuous-wave spin-lock pulse and a reverse AHP tip-up pulse (rAHP), which is designed to be robust to B1 and B0 inhomogeneities 9. Image acquisition is performed under free-breathing, and the imaging slice is adjusted in real time with the diaphragm navigator (dNav) to compensate for the through-plane respiratory motion 7,8. To avoid the influence of IR on the dNav signal, a slice-selective IR (SSIR) is performed in the location of dNav along with IR. After the acquisition, the PCA-based groupwise registration is adopted to correct in-plane motion among the multi-contrast images10. For parameter quantification, dictionary matching is performed, where the dictionary is generated with Bloch simulations for a range of T1, T2, and T1ρ values as well as B1 factors to account for the inhomogeneous B1 fields. The T1 and T2 relaxations during the long AHP and rAHP pulses in the T1ρ prep are considered in the simulation.Experiments
The proposed sequence was evaluated in phantoms made of different concentrations of agarose and Gadolinium-based contrast agent on a 3T United Imaging scanner. Reference T1, T2, and T1ρ values were respectively obtained using IR spin-echo (IRSE), multi-echo spin-echo (MESE), and T1ρ prep gradient echo (T1ρ-GRE) sequences 9. To test the influence of heart rates on the proposed technique, it was performed with simulated heart rates from 40 to 120bpm with a step of 20 bpm. The conventional MOLLI 11, T2-prep bSSFP (T2-bSSFP) 12, and T1ρ-prep bSSFP(T1ρ-bSSFP) 9 were also performed at 80 bpm for comparison. The imaging parameters were: FOV=320 × 280mm2, in-plane resolution=2.02×1.82mm2, thickness=8mm, TR/TE/flip angle=3.01ms/1.5ms/35°, bandwidth=1200 Hz/px, GRAPPA acceleration factor=2.Six healthy subjects (age=24±2 years, 5 males) were imaged using the proposed technique at three short-axis slices with the same imaging parameters to the phantom imaging. The breath-hold MOLLI, T2-bSSFP and T1ρ-bSSFP were also performed for comparison. The American Heart Association’s (AHA) 16-segment model was adopted for segment-wise analysis 13. The mean and SD were calculated for each segment and all three acquired slices over all subjects.
Results
As the phantom results shown in Fig. 2, all the parameters estimated with the proposed sequence had a good agreement with the reference methods and showed robustness to the wide range of simulated heart rates, except that the short T1 values tend to be overestimated at low heart rates, which also leads to inaccurate estimation of T2 and T1ρ of that tube. Evaluating at heart rate of 80bpm, the conventional MOLLI, T2-bSSFP, and T1ρ-bSSFP had higher estimation errors than the proposed method.Fig. 3 shows the mapping results of a representative subject. The proposed technique achieved similarly good mapping quality to the breath-hold techniques without obvious motion artifacts. The Bullseye plots of the mean and SD of the measurements averaged over the healthy subjects are shown in Fig. 4. The global T1, T2 and T1ρ values measured by the proposed method and the breath-hold techniques were respectively (mean ± SD): 1177±53ms vs. 1138±34ms, 40.1±2.7ms vs. 45.8±2.6ms, and 41.0±3.6ms vs. 51.3±3.9ms.
Discussion
The proposed technique provided T1, T2, and T1ρ maps simultaneously in a single acquisition of 16 heartbeats under free-breathing. Prospective through-plane and retrospective in-plane motion correction were performed to mitigate respiratory motion artifacts. Dictionary matching was performed to account for subjective heart rates and B1 inhomogeneities. The phantom results indicated better accuracy of the proposed technique than the conventional single-parameter mapping techniques. Similarly good mapping quality was observed for the proposed technique, compared with the conventional breath-hold methods, while the measured T1 was higher than MOLLI, and T2 and T1ρ were lower than the T2- and T1ρ-bSSFP.Acknowledgements
No acknowledgement found.References
1. Warnica W, Al-Arnawoot A, Stanimirovic A, Thavendiranathan P, Wald RM, Pakkal M, et al. Clinical Impact of Cardiac MRI T1 and T2 Parametric Mapping in Patients with Suspected Cardiomyopathy. Radiology. 2022;(10).2. Stoffers RH, Madden M, Shahid M, Contijoch F, Solomon J, Pilla JJ, et al. Assessment of myocardial injury after reperfused infarction by T1ρ cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19(1):1–10.
3. Thompson EW, Kamesh Iyer S, Solomon MP, Li Z, Zhang Q, Piechnik S, et al. Endogenous T1ρ cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2021;23(1):1–9.
4. Jerosch-Herold M, Coelho-Filho O. Cardiac MRI T1 and T2 Mapping: A New Crystal Ball? Radiology. 2022;327–8.
5. Velasco C, Cruz G, Lavin B, Hua A, Fotaki A, Botnar RM, et al. Simultaneous T1, T2, and T1ρ cardiac magnetic resonance fingerprinting for contrast agent–free myocardial tissue characterization. Magn Reson Med. 2022;87(4):1992–2002.
6. Henningsson M. Cartesian dictionary-based native T1 and T2 mapping of the myocardium. Magn Reson Med. 2022;87(5):2347–62.
7. Basha TA, Roujol S, Kissinger K V., Goddu B, Berg S, Manning WJ, et al. Free-breathing cardiac MR stress perfusion with real-time slice tracking. Magn Reson Med. 2014;72(3):689–98.
8. Guo R, Cai X, Kucukseymen S, Rodriguez J, Paskavitz A, Pierce P, et al. Free-breathing simultaneous myocardial T1 and T2 mapping with whole left ventricle coverage. Magn Reson Med. 2021;85(3):1308–21.
9. Qi H, Lv Z, Hu J, Xu J, Botnar R, Prieto C, et al. Accelerated 3D free-breathing high-resolution myocardial T1ρ mapping at 3 Tesla. Magn Reson Med. 2022;(July):2520–31.
10. Huizinga W, Poot DHJ, Guyader JM, Klaassen R, Coolen BF, Van Kranenburg M, et al. PCA-based groupwise image registration for quantitative MRI. Med Image Anal. 2016;29:65–78.
11. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified look-locker inversion recovery (MOLLI) for high-resolution T 1 mapping of the heart. Magn Reson Med. 2004;52(1):141–6.
12. Huang TY, Liu YJ, Stemmer A, Poncelet BP. T2 measurement of the human myocardium using a T 2-prepared transient-state trueFISP sequence. Magn Reson Med. 2007;57(5):960–6.
13. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. J Cardiovasc Magn Reson. 2002;4(2):203–10.
Figures
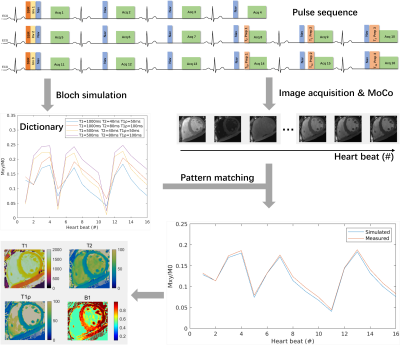
Fig. 1 Pulse sequence and framework for the proposed Multimapping technique (Multi-Map) with Cartesian sampling and dictionary matching. The parameters of preparation modules are as follows: IR TIs={240, 240, 240}ms, T2-prep TEs={25, 40, 55}ms, and T1ρ-prep spin-lock times (TSL)={16, 30, 50}ms at SL frequency=350 Hz.
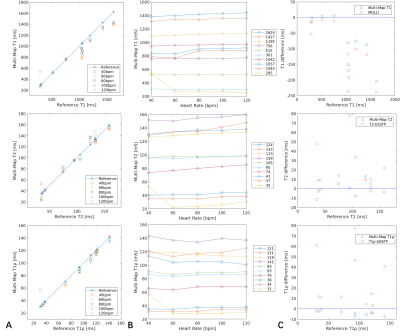
Fig. 2 Phantom results comparing the proposed method (Multi-Map) with the reference methods. The measured T1, T2, and T1ρ values at different heart rates and the corresponding errors are shown in A, B. C: Measurement errors of the proposed method and the conventional MOLLI, T2-bSSFP, and T1ρ-bSSFP at 80 bpm.
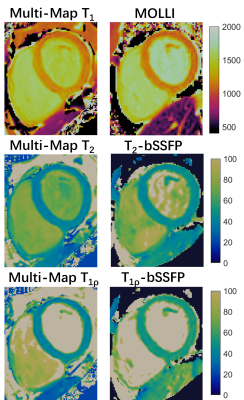
Fig. 3 T1, T2, and T1ρ maps from a health subject using the proposed free-breathing Multi-Map technique and the traditional breath-hold MOLLI, T2-bSSFP, and T1ρ-bSSFP methods.
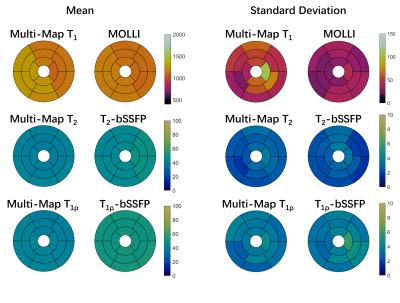
Fig. 4 Sixteen-segment AHA Bullseye plots showing mean and SD of T1, T2, and T1ρ values averaged across healthy volunteers for the proposed free-breathing Multi-Map technique and the traditional breath-hold MOLLI, T2-bSSFP, and T1ρ-bSSFP techniques.