0170
Evaluation of Cardiac Microstructure in Patients with Severe Aortic Stenosis Using Diffusion Tensor MRI with Submillimeter Resolution1Cardiovascular Research Center, Massachusetts General Hospital, Boston, MA, United States, 2A.A Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Giessen, Germany, 4Cardiovascular Innovation Research Center, Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH, United States, 5Health Sciences and Technology Program, Harvard - Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Keywords: Myocardium, Diffusion Tensor Imaging
This study describes a method to acquire submillimeter resolution diffusion tensor MRI scans of the human heart in vivo. Imaging was performed in healthy controls and patients with severe high flow-high gradient aortic stenosis. The images were processed to generate helix angle maps of cardiomyocyte orientation and compared. The range of helix angles across the myocardium was higher in subjects with aortic stenosis and the relative helix angle slope was also increased in patients with aortic stenosis.Introduction
Diffusion tensor MRI (DTI) of the human heart in vivo is typically performed with an in-plane resolution of 2.5x2.5mm2. However, this level of resolution can result in as few as five voxels across the wall of the heart, even when it is imaged in systole. The transmural slope of helix angle (HA) is an important metric of cardiac microstructure and has been reported to increase in patients with severe aortic stenosis (AS).1 It remains unclear, however, whether the low resolution of standard DTI acquisitions, which particularly under-resolve the thinner wall of the left ventricle (LV) in healthy volunteers, may be affecting these results. The aims of this study were, therefore, to develop an in vivo DTI acquisition technique with submillimeter resolution and to use this to evaluate the transmural patterns of HA in healthy subjects and patients with severe AS.Methods
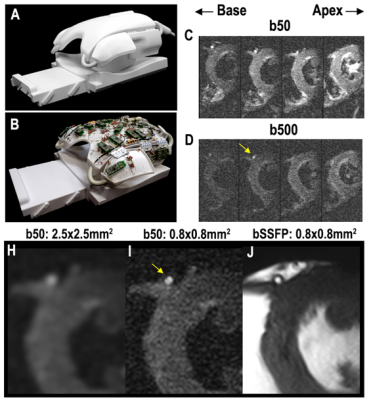
DTI of the heart was performed on a 3T scanner with 80mT/m gradients (Primsa, Siemens) using a custom-built 64-element cardiac array.2 A free breathing M2-compensated diffusion-encoded spin echo EPI sequence, with a spatially tailored 2D excitation pulse, was used. Parameters included: FOV 23.7cm, phase FOV 24%, MTX 286x70, slice thickness 8mm, resolution 0.85x0.85mm2, chemical fatsat, echoplanar imaging readout, TR = 12RR, TE=76ms, b = 50, 500 s/mm2, 12 directions, 12 averages. Images were acquired in mid-late systole. The selected inner volume (FOV) was centered over the septum of the LV which resulted in the lateral wall of the LV being truncated in some subjects. Healthy volunteers (n=16) and subjects with severe high flow-high gradient AS (n=8) were imaged. Respiratory gating of the free-breathing data was performed, as previously described,3 using low-rank modeling and a multitasking motion-compensation framework. Further processing of the data was performed in Matlab (Natick MA) to produce maps of mean diffusivity (MD), fractional anisotropy (FA), and cardiomyocyte helix angle (HA). Analysis of the data was limited to the septum, which is an emerging convention, since the potential for susceptibility artifacts and distortion is lowest in this region. The transmural thickness, HA range, HA slope, and mean values of MD and FA were calculated. The transmural range and slope of HA from endocardium to epicardium was measured along multiple radial projections, with each pixel classified either by percent transmural depth from the endocardium or absolute distance (mm) from the endocardium. The data followed a normal distribution and significance was, therefore, determined using an unpaired t-test comparing AS to control, with p<0.05 being significant. Metrics are expressed as mean ± standard deviation.Results
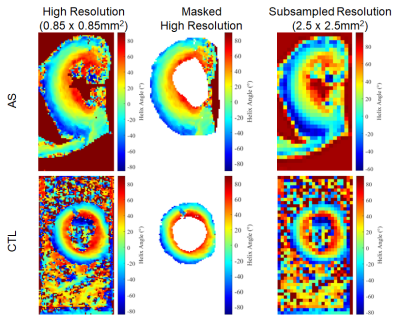
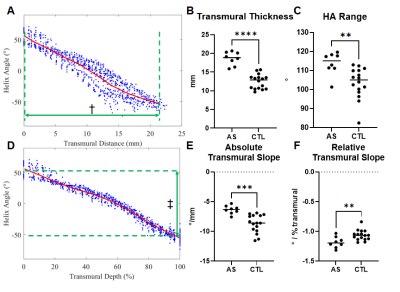
High quality diffusion weighted images could be acquired in all subjects and small structures, such as the left coronary artery and myocardial trabeculations, could be well resolved (Figure 1). Image quality was highest in the mid-portion of the LV and in each subject 3-5 slices at the mid-ventricular level were analyzed (Figure 1). Representative HA maps in a control and AS subject are shown in Figure 2 at the acquired resolution (0.85x0.85mm2) and subsampled down to the standard resolution of 2.5x2.5mm2. AS subjects had a significantly thicker septum (Figure 3) relative to healthy subjects (18.5±3.1mm vs 12.4±2.6mm), but even in the healthy controls >10 pixels could be imaged from endocardium to epicardium at the resolution of 0.85mm. The subjects with severe AS had a greater HA range (112±15° vs 104±18°) and a higher HA slope, when pixel position was expressed in terms of % transmural depth (-1.17±0.16 °/% vs -1.07±0.18 °/%), as shown in Figure 3. There was no significant difference in mean MD and FA values between AS and control subjects.Conclusions
DTI of the human heart can be performed in vivo with submillimeter in-plane resolution using advanced multi-element receive coils and inner volume selection using a spatially tailored excitation pulse. This resolution minimizes volume averaging in the subendocardium and subepicardium and allows the full transmural range of HA to be sampled. In agreement with a recent report,1 our data suggest that the transmural range and relative slope of HA are increased in subjects with severe AS.Acknowledgements
No acknowledgement found.References
1. Gotschy A, von Deuster C, Weber L, Gastl M, Schmiady MO, van Gorkum RJH, Stimm J, von Spiczak J, Manka R, Kozerke S and Stoeck CT. CMR Diffusion Tensor Imaging Provides Novel Imaging Markers of Adverse Myocardial Remodeling in Aortic Stenosis. JACC Cardiovasc Imaging. 2021;14:1472-1474.
2. Etzel R, Mekkaoui C, Ivshina ES, Reese TG, Sosnovik DE, Hansen SJD, Ghotra A, Kutscha N, Chemlali C, Wald LL, Mahnken AH and Keil B. Optimized 64-channel array configurations for accelerated simultaneous multislice acquisitions in 3T cardiac MRI. Magn Reson Med. 2021;86:2276-2289.
3. Nguyen CT, Christodoulou AG, Coll-Font J, Ma S, Xie Y, Reese TG, Mekkaoui C, Lewis GD, Bi X, Sosnovik DE and Li D. Free-breathing diffusion tensor MRI of the whole left ventricle using second-order motion compensation and multitasking respiratory motion correction. Magn Reson Med. 2021;85:2634-2648.
Figures