0167
A Machine Learning-Based Prediction of RF Transfer Function of Active Implantable Medical Devices in Homogeneous and Heterogeneous Tissue1Lanzhou University, Lanzhou, China
Synopsis
Keywords: Bioeffects & Magnetic Fields, Safety
Both the Tier-3 and Tier-4 methods proposed in ISO/TS 10974 for assessing the radiofrequency safety of active implantable medical devices have limitations in practice. The transfer function proposed in the Tier-3 method can only be measured individually for each implant and only for homogeneous tissue environments, while Tier-4 requires significant high-speed computing resources. In this study, machine learning algorithms are proposed to predict the transfer function both in homogeneous and heterogeneous tissue environment. The results show that this approach is feasible and performs well in the prediction task.Introduction
The issue of safety risks for patients wearing active implantable medical device (AIMD) undergoing magnetic resonance imaging (MRI) examinations has been a long-standing concern. A four-Tier approach is proposed in ISO/TS 109741 to assess the heating of the tissue surrounding the implant, with the Tier-3 and Tier-4 approaches being suitable for AIMDs with long electrical lengths. Whereas there are still many limitations or simplifications when performing these methods. Tier-4 approach requires full-wave electromagnetic modelling of implant, human and radiofrequency (RF) coil, which is notoriously time consuming and high-powered computing clusters is needed2. Tier-3 transforms tangential incident E-field into the temperature rise of the tissue near the implant electrode by means of the implant's RF transfer function. However, the current mainstream methods for measuring transfer function greatly simplify the human tissue in the vicinity of the implant and use a homogeneous simulation fluid as a substitute3-5, thus AIMDs with relatively complex implant environments are not suitable for these methods in most cases. More importantly, both Tier-4 and the current methods for measuring transfer functions can only be evaluated on a case-by-case basis for each implant, which can be quite time-consuming.With these problems in mind, we propose machine learning algorithms that can predict the RF transfer function of AIMDs both in homogeneous and heterogeneous environments based on their geometric parameters and tissue distribution around the AIMDs. In this way, not only a rapid and effective assessment of the RF transfer function can be completed, but the impact of the complicated tissue environment on the transfer function is also taken into accounts in the assessment process.
Method
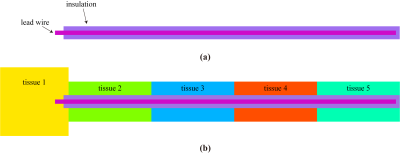
A generic AIMD model was constructed as shown in Figure 1(a), several AIMD models with different geometrical parameters of length 400~700 mm (step: 50mm), electrode length 1~5 mm (step: 1 mm), lead wire radius 0.1~0.4 mm (step: 0.1 mm), and insulation thickness 0.1~0.4 mm (step: 0.1 mm) were generated. In addition, in the case of heterogeneous, the tissue was modeled at 10 cm intervals along the length of the implant (3 cm at the tip of the electrode), as shown in Figure 1(b).Finite-difference time domain (FDTD) simulations were performed in Sim4Life v7.0 (ZMT Zurich MedTech, Zurich, Switzerland) to model the RF transfer function of the AIMD. Unlike the conventional method of measuring the transfer function by piece-wise excitation, we set up a hard current source near the implant electrode based on the reciprocity principle5 and current sensors were placed at 5 mm intervals around the implant to capture the current distribution and obtain the transfer function. In the homogeneous circumstance, the phantom was modeled as tissue material with conductivity of σ = 0.4~1.2 S/m (in steps of 0.2 S/m), and permittivity of εr = 10~80 (in steps of 10). In the heterogeneous case, each segment of tissue as shown in Figure 1(b) was set to a randomly generated tissue category from Table 1. The operating frequency was at 128 MHz for 3T MRI. Wave number k and input impedance Z are used to fit the transfer function curve S(l), the formula is as follows6:
$$S(l)=C\cdot(i/2Z)\cdot\frac{sink(L/2-l)}{cosL/2}$$
where C is a constant factor, and L is the length of AIMD.
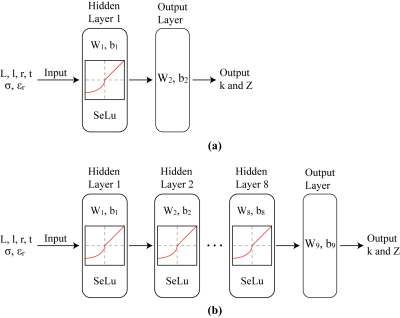
As shown in Figure 2, feedforward neural networks based on Keras sequential7 were designed for homogeneous and heterogeneous cases. The 532 homogeneous scenarios and 592 heterogeneous scenarios were divided randomly into training set (60%), validation set (20%), and testing set (20%), respectively. AIMD length L, electrode length l, lead wire radius r and insulation thickness t, as well as the conductivity σ and permittivity εr of tissues are used as the inputs for the neural network. The output would be wave number and input impedance. Predictive performance was evaluated with the correlation coefficient (R), the SeLu activation functions were used in the hidden layers. Batch normalization is applied to solve the problem of covariance shift. Hyperparameter optimization was conducted using SGD8 in homogeneous network and Adam9 in heterogeneous network.
Results
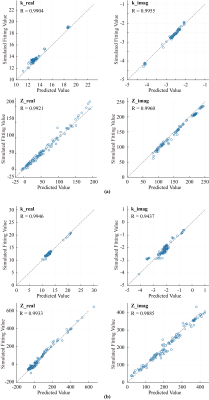
Figure 3(a) shows the performance of the proposed machine learning algorithm in predicting k and Z from geometrical parameters of AIMD and electronic parameters of tissue in homogeneous tissue environments.From the results, the designed algorithm is able to predict well for k and Z, testing performance is indicated with an average R ≈ 0.99. And Figure 3(b) is for heterogeneous tissue environment, with an average R ≈ 0.98.
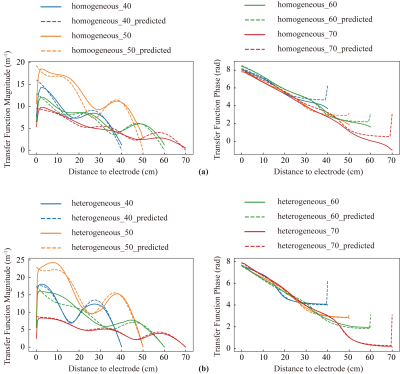
Comparison of predicted and simulated transfer functions for lengths of 40 cm, 50 cm, 60 cm, and 70 cm in homogeneous and heterogeneous tissue environment is shown in Figure 4.
Conclusion
A machine learning-based evaluation method has been proposed in this study to enable a rapid and accurate prediction of the AIMD transfer function. As a preliminary study, the results show that the proposed method can be used as an alternative to traditional numerical and experimental transfer function assessment. With the proposed approach, inherent disadvantages of numerical simulations (e.g., expensive computational cost) and experimental measurements (e.g., limitation in measuring under heterogeneous tissue conditions) may be overcome. Moreover, the proposed approach can be applied to both homogeneous and heterogeneous implantation scenarios, allowing a more flexible implementation under different clinical tissue environments.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No.6210010061). The authors would like to thank Zurich MedTech (ZMT, Zurich, Switzerland) for providing Sim4Life for scientific use.References
1. ISO/TS. 10974: Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device. Geneva: International Organization for Standardization. 2018.
2. Nguyen BT, Pilitsis J, Golestanirad L. The effect of simulation strategies on prediction of power deposition in the tissue around electronic implants during magnetic resonance imaging. Physics in Medicine & Biology. 2020;65(18):185007.
3. Zastrow E, Capstick M, Kuster N. Experimental system for RF-heating characterization of medical implants during MRI. ISMRM; 2016.
4. Tokaya JP, Raaijmakers AJE, Luijten PR, et al. MRI-based transfer function determination through the transfer matrix by jointly fitting the incident and scattered field. Magnetic Resonance in Medicine. 2020;83(3):1081-1095.
5. Feng S, Qiang R, Kainz W, et al. A Technique to Evaluate MRI-Induced Electric Fields at the Ends of Practical Implanted Lead. IEEE Transactions on Microwave Theory and Techniques. 2015;63(1):305-313.
6. King RWP, Trembly BS, Strohbehn JW. The Electromagnetic Field of an Insulated Antenna in a Conducting Or Dielectric Medium. IEEE Transactions on Microwave Theory and Techniques. 1983;31(7):574-583.
7. Haghighat E, Juanes R. SciANN: A Keras/TensorFlow wrapper for scientific computations and physics-informed deep learning using artificial neural networks. Computer Methods in Applied Mechanics and Engineering. 2021;373:113552.
8. Netrapalli P. Stochastic Gradient Descent and Its Variants in Machine Learning. Journal of the Indian Institute of Science. 2019;99(2):201-213.
9. Bock S, Weiß M. A Proof of Local Convergence for the Adam Optimizer. 2019 International Joint Conference on Neural Networks (IJCNN); 2019.
Figures