0145
Hyperpolarized [1-13C] Gluconolactone Can Visualize TERT Silencing Directly or via the Upstream Transcriptional Factor GABPB1 in Glioblastoma1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Department of Neurological Surgery, UCSF, San Francisco, CA, United States
Synopsis
Keywords: Tumors, Brain, Glioblastoma, GBM, TERT, GABP, imaging, MRI, MRS, NMR
TERT promoter mutation is a genetic hallmark of glioblastoma, and targeting TERT or its upstream transcriptional factor GABPB1 are considered ideal therapeutic targets. Accordingly, establishing reliable imaging modalities that will enable monitoring of TERT silencing are needed as early indicators of target engagement and response to these molecular targeting therapies. Here, we demonstrate that using 13C MRS to monitor the metabolism of hyperpolarized δ-[1-13C] gluconolactone via the pentose phosphate pathway to 6-phosopho-[1-13C]gluconate (6PG) provides such a noninvasive imaging readout of TERT or GABPB1 silencing in glioblastoma.Introduction
Glioblastoma (GBM) remains the most aggressive and treatment-resistant malignant primary intracranial tumor with a very poor prognosis of 15 months. Upregulation of TERT expression due to TERT promoter mutations is a prominent genetic hallmark of GBM, present in 80% of GBM patients. As a result, TERT and its upstream tumor-specific transcriptional factor GABPB1 are being investigated as potential therapeutic targets 1 2. However, imaging methods that can detect TERT expression and its inhibition by emerging therapies are limited. In our previous study, silencing TERT or GABPB1 in GBM resulted in consistent and significant reduction of NADPH and GSH in line with inhibition of flux via the pentose phosphate pathway (PPP) 3. In the present study, we asked whether hyperpolarized [1-13C] gluconolactone, which has been reported as a probe for the PPP 4,5, can monitor TERT silencing in GBM in cells and in tumors in vivo.Methods
Cell models: U251 cell lines stably expressing shRNA silencing TERT or GABPB1 were compared to controls (U251shCtrl, U251shTERT and U251shB1 respectively).Hyperpolarized 13C-MRS in live cells: δ-[1-13C] gluconolactone was synthesized and polarized as previously described 5. 2 M δ-[1-13C] gluconolactone was dissolved in 3:1 water: glycerol and mixed with 15mM trityl radical OX063. After polarization, samples were dissolved in 3.9ml phosphate-buffered saline. HP δ-[1-13C] gluconolactone was injected into ~108 live cells in a 10mm NMR tube to a final concentration of 8mM. 13C-MRS spectra were acquired every 3s for 300s on a Varian 500MHz NMR spectrometer using a 13deg pulse and data was analyzed using MestReNova.
Hyperpolarized 13C-MRS in vivo: For in vivo studies, the cells described above were injected into male athymic immunodeficient rat brains. All measurements were performed on a horizontal 3 T pre-clinical scanner (Bruker, Germany), equipped with a quadrature 1H-13C volume coil (Neos-Biotech, Spain). Tumor growth was monitored via axial T2-weighted images. Hyperpolarized 13C studies were performed following injection of 2.5ml HP δ-[1-13C] gluconolactone via a tail-vein catheter over 12 s. Data were acquired using a spectral-spatial echo planar spectroscopic imaging (EPSI) sequence with final resolution of 5.375x5.375x8mm3 (TR=3s/NR=20/FA=15.2 degrees on 6PG and 3.4 degree on δ-[1-13C] gluconolactone). For the EPSI data, SNR of each voxel spectrum at every time point was improved using tensor denoising 6,7, and spectra were analyzed using in-house Matlab (Mathwork, MA) script for all post-processing and spectral quantification procedures.
Statistical analysis: All results represent mean±STD. One-Way ANOVA with multiple comparisons was used to assess the statistical significance of differences with p<0.05 considered significant.
Results
First, we confirmed our previous findings that the NADPH/NADP+ ratio is significantly reduced when TERT or GABPB1 are silenced in our cells (Fig. 1A). Next we checked the expression of PGLS, which is an essential enzyme of the PPP involved in 6-PG production (Fig 1B), and demonstrated that the expression of PGLS was reduced by TERT or GABPB1 silencing in our U251 cells (Fig. 1C). Based on these findings, and our previous observation that GSH is correlated with TERT expression3, we examined the utility of hyperpolarized δ-[1-13C] gluconolactone for monitoring the PPP and its modulation by TERT silencing in our cells and tumors. A representative spectral array shows the dynamic conversion of hyperpolarized δ-[1-13C] gluconolactone to 6PG (Fig. 2A), and quantification of the data demonstrated that hyperpolarized 6PG normalized to the sum of δ- and γ-[1-13C] gluconolactone was significantly reduced in TERT or GABPB1-silenced U251 cells compared to controls (Fig. 2B) with a positive correlation between TERT expression and hyperpolarized 6PG confirmed by linear regression analysis (Fig. 2C). Next, we probed tumors in vivo. As illustrated in Figure 3A and B, we could clearly detect a drop in 6PG production in tumors that had TERT expression silenced. This was further confirmed by quantification of the results which showed a significant drop in 6PG production in TERT or GABPB1 silenced tumors compared to control tumors (Fig. 3C).Conclusions
We successfully visualized TERT or GABPB1-associated changes in dynamic PPP metabolism in GBM models in live cells and in vivo. Hyperpolarized δ-[1-13C] gluconolactone can potentially serve as a useful tool for monitoring tumor burden and response to TERT-targeted therapy for GBM in the clinic.Acknowledgements
This work was supported by R01CA172845, NIH P01CA118816, UCSF LOGLIO collective and UCSF NICO project. The authors also acknowledge support from NIH P41EB013598.References
1. Mancini A, Xavier-Magalhães A, Woods WS, et al. Disruption of the β1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer Cell. 2018; 34(3):513-528.e518.
2. Amen AM, Fellmann C, Soczek KM, et al. Cancer-specific loss of TERT activation sensitizes glioblastoma to DNA damage. Proc Natl Acad Sci U S A. 2021; 118(13).
3. Minami N, Hong D, Stevers N, et al. Imaging biomarkers of TERT or GABPB1 silencing in TERT-positive glioblastoma. Neuro Oncol. 2022.
4. Batsios G, Taglang C, Cao P, et al. Imaging 6-Phosphogluconolactonase Activity in Brain Tumors In Vivo Using Hyperpolarized δ-[1-(13)C]gluconolactone. Front Oncol. 2021; 11:589570.
5. Moreno KX, Harrison CE, Merritt ME, Kovacs Z, Malloy CR, Sherry AD. Hyperpolarized δ-[1-(13) C]gluconolactone as a probe of the pentose phosphate pathway. NMR Biomed. 2017; 30(6).
6. Brender JR, Kishimoto S, Merkle H, et al. Dynamic Imaging of Glucose and Lactate Metabolism by (13)C-MRS without Hyperpolarization. Sci Rep. 2019; 9(1):3410.
7. Chen HY, Autry AW, Brender JR, et al. Tensor image enhancement and optimal multichannel receiver combination analyses for human hyperpolarized (13) C MRSI. Magn Reson Med. 2020; 84(6):3351-3365.
Figures
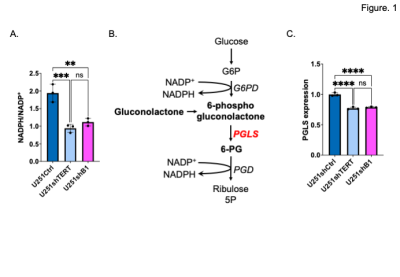
A. NADPH/NADP+ level comparing U251shCtrl, U251shTERT, and U251shB1.
B. Diagram of the pentose phosphate pathway showing its role in NADPH production.
C. Relative mRNA expression of PGLS comparing U251shCtrl, U251shTERT and U251shB1 (shGABPB1).
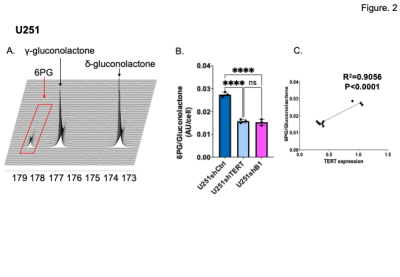
A. Representative spectral array of 13C spectra following injection of [1-13C] δ-gluconolactone into U251shCtrl cells.
B. The AUC of [1-13C]6PG/total [1-13C]gluconolactone comparing U251shCtrl, U251shTERT, and U251shB1 cells.
C. [1-13C]6PG/total [1-13C]gluconolactone levels in U251shCtrl, U251shTERT, and U251shB1 cells as a function of TERT expression.
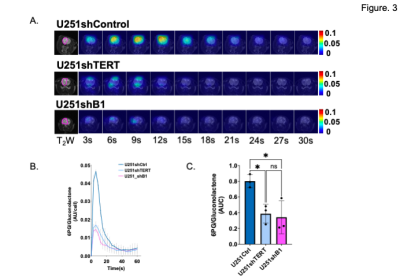
A. Dynamic hyperpolarized 13C EPSI data set of [1-13C]6PG acquired from one slice from corresponding tumor voxels in T2-weighted images at 3 s temporal resolution.
B. Average dynamic 6PG to total [1-13C]gluconolactone ratio of tumor voxel from each tumor model.
C. AUC of [1-13C]6PG to total [1-13C]gluconolactone comparing U251shCtrl, U251shTERT, and U251shB1.