0140
Transmembrane water-efflux rate measured by water exchange DCE-MRI: a sensitive and specific biomarker of Aquaporin-4 in gliomas1Key Laboratory of Biomedical Engineering of Ministry of Education, College of Biomedical Engineering and Instrument Science, Zhejiang University, Hangzhou, China, 2School of Medicine, Zhejiang University, Hangzhou, China, 3MR Collaboration, Siemens Healthcare, Shanghai, China, 4Department of Radiology, Qilu Hospital of Shandong University, Jinan, China, 5Department of Neurosurgery, Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China, 6Shandong National Center for Applied Mathematics, Shandong University, Jinan, China, 7College of Biomedical Engineering and Instrument Science, Zhejiang University, Hangzhou, China
Synopsis
Keywords: Tumors, Brain, Glioma, Aquaporins-4, transmembrane water-efflux rate, therapy-resistant.
The water-selective channel aquaporin-4 (AQP4) contributes to the migration and proliferation of gliomas, and to their resistance to therapy. Here, we show, in glioma animal models, and in glioma patients, that transmembrane water-efflux rate is a sensitive and specific biomarker of AQP4 expression and can be measured via dynamic-contrast-enhanced magnetic resonance imaging. Water-efflux rates correlated with changes in the heterogeneity of intratumoural and intertumoural AQP4 in human gliomas and following treatment with the AQP4 inhibitor TGN020. Regions with low water-efflux rates contained higher fractions of stem-like slow-cycling cells and therapy-resistant cells, suggesting that maps of water-efflux rates could be used to identify gliomas that are resistant to therapies.
Introduction
Glioma is the most prevalent primary central nervous system (CNS) malignancy in adults 1. Aquaporin channel 4 (AQP4) plays an important role in glioma pathology 2–4, including facilitating glioma infiltration into the brain and being one of the earliest indicators of glioma transformation and treatment resistance. Currently, traditional biopsy is the ‘gold-standard’ approach to characterize the expression levels of AQP4, but lacks spatial information. In this study, we show that imaging AQP4 expression at high spatial resolution via MRI is achievable. We show that the intracellular-to-extracellular water-efflux-rate constant kio, a physical parameter characterizing the membrane permeability property of cells obtained from water-exchange DCE-MRI (ref 5 in Fig. 1b), is a non-invasive and accurate MRI imaging biomarker of AQP4 expression in glioma.Methods
Patients, Water-exchange MRI, and kio-guided Stereotactic BiopsyGlioma patients were recruited according to inclusion criteria of the Institutional Review Board (IRB) of Shandong Provincial Hospital, Shandong First Medical University. Water-exchange DCE-MRI (Fig 1) were performed and analyzed following the same protocol as ref 6 one or two days before stereotactic biopsy procedure. The obtained kio map was registered to the structural MRI for stereotactic biopsy planning. In each patient, multiple biopsy samples were selected with varying kio values (Fig. 1b). The AQP4 expression level of “point-to-point” were further obtained from immunohistochemistry (IHC).
Glioma Rat Model and AQP4 inhibition
The subcutaneous glioma model was used by injecting the C6 glioma cell lines in the right leg of Sprague Dawley rats. Then, the animals were taken for water-exchange DCE-MRI on a 7.0T MRI and were fixed with 4% PFA immediately after MRI for IHC staining of AQP4. For pharmacological inhibition of AQP4, Each animal was intravenously injected with TGN020 (3 mg/kg) 15 mins before water-exchange DCE-MRI, ensuring that TGN020 maintained a steady uptake 7,8.
Results and Discussion
kio precisely detects AQP4 expression in human gliomasWater-exchange DCE-MRI revealed intratumoural heterogeneity of kio in human glioma (Fig. 2). The MRI-guided stereotactic biopsy enabled us to compare kio directly with the biopsy histology in a “point-to-point” way. From visual inspection, the sample with kio = 10.0 s-1 shows much higher AQP4 expression than that with kio < 0.5 s-1 in both IHC and immunoelectron microscopy. We further quantified the AQP4+ fraction in each biopsy and find a significant linear correlation (R = 0.97, p < 0.0001) between kio and the fraction of AQP4+ cells using all 45 biopsies subsequently (Fig. 2f). Linear regression analysis generated:
kio = 14.1 s-1 * AQP4+ % + 0.2 s-1
suggesting a greater AQP4-related kio (14.9 s-1) than non-AQP4-related kio (i.e., kio baseline, 0.2 s-1). and kio is dominated by the AQP4-regulated pathway in human glioma. Such linear relation between kio and AQP4 expression still holds in the multiple biopsy samples acquired for the same patients as illustrated in Fig.3.
kio is a specific AQP4 biomarker
To further validate water-exchange DCE-MRI as a specific imaging method related to AQP4 expression in vivo, we performed AQP4 inhibition with TGN020 in the rat subcutaneously glioma model (Fig. 4a). Glioma tumor treated with TGN020 exhibited a clear reduction in kio values, as shown in the kio maps (Fig. 4b). In addition, whole-tumor-averaged kio decreased significantly from 6.3 ± 0.6 s-1 (saline) to 3.5 ± 0.7 s-1 (TGN020) (by 43%, n = 9, Fig. 4c). The qualitative results demonstrated the sensitivity and specificity of kio as an AQP4 expression surrogate in gliomas.
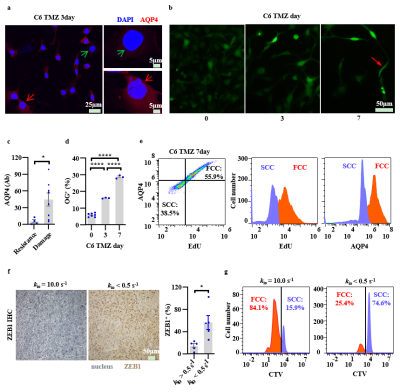
Low kio (AQP4) reflects treatment-resistance glioma phenotypes
Here, we found the low-kio (i.e., low-AQP4) cells could potentially represent therapy-resistant cell subtypes in glioma. Low-AQP4 Cells show nuclei damaged by TMZ while high-AQP4 show TMZ-resistance with more intact nucleus structure (Fig. 5a, 5c). On the other hand, during TMZ treatment we also observed a simultaneous increase in the population of quiescent, slow-cycling cells (SCCs), which are more TMZ-resistant than fast-cycling cells (FCCs) (Fig. 5b, 5d). The further flow cytometry tests demonstrated the SCCs (low EdU, 5-Ethynyl-2’-deoxyuridine, a biomarker of cells proliferation) having lower AQP4 expression than FCCs (Fig. 5e) and vice versa. The kio values also show significantly negative correlations with SCCs fractions, consistent with the findings that lower kio (i.e., AQP4) is associated with more SCCs along with lower proliferation and represents the therapy-resistant phenotype (Fig. 5e, f). In human glioma, low-AQP4 sample is also associated with higher expression of ZEB1(zinc-finger-enhancer binding protein 1, therapy-resistance biomarker, Fig. 5f). And the primary cells from the low-kio biopsy samples also show much higher fraction of SCC phenotypes (CTV+) than that from high-kio sample, further suggesting low kio could represent therapy-resistant glioma phenotype.
Conclusion
In summary, we have provided a non-invasive image biomarker, kio, for the detection and mapping of AQP4 in gliomas in vivo. More importantly, we found that lower-kio cells show higher therapy resistance, suggesting that AQP4 maps can be used to assess therapy resistance in glioma. Our approach could be used to diagnose and assess gliomas via widely available MRI equipment, and might improve the precise assessment and management of gliomas in humans.Acknowledgements
The study was supported by the National Natural Science Foundation of China (NSFC) (grants 81873894 and 82172050, to R.B.), the Natural Science Foundation of Zhejiang Province, China (grant LR20H180001, to R.B.), the Taishan Scholars Program (No. tsqn20161070, to Y.L.), and the Natural Science Foundation of Shandong Province (grant ZR2019HM067, to Y.L.). We appreciate discussions and constructive comments from Peter J. Basser at the National Institutes of Health, Jonathan Polimeni at Harvard Medical School and Massachusetts General Hospital, and Zengjing Chen at the Department of Mathematics, Shandong University. We also thank the support from the MOE Frontier Science Center for Brain Science & Brain-Machine Integration, Zhejiang University.
References
[1] Omuro, A.; DeAngelis L M. Glioblastoma and Other Malignant Gliomas. Jama. 2013;310(17):1842–1850.
[2] Sun D-P, Lee Y-W, Chen J-T, et al. The Bradykinin-BDKRB1 Axis Regulates Aquaporin 4 Gene Expression and Consequential Migration and Invasion of Malignant Glioblastoma Cells via a Ca(2+)-MEK1-ERK1/2-NF-κB Mechanism. Cancers. 2020;12(3):667.
[3] Nico B, Mangieri D, Tamma R, et al. Aquaporin-4 contributes to the resolution of peritumoural brain oedema in human glioblastoma multiforme after combined chemotherapy and radiotherapy. European Journal of Cancer, Elsevier Ltd. 2009;45(18):3315–3325.
[4] Papadopoulos M C, Verkman A S. Aquaporin water channels in the nervous system. Nature Reviews Neuroscience. 2013;14(4):265–277.
[5] Bai R, Wang B, Jia Y, et al. Shutter-Speed DCE-MRI Analyses of Human Glioblastoma Multiforme (GBM) Data. Journal of magnetic resonance imaging : JMRI. United States: 2020;52(3):850–863.
[6] Bai R, Springer C S, Plenz D, et al. Fast, Na+/K+ pump driven, steady-state transcytolemmal water exchange in neuronal tissue: A study of rat brain cortical cultures. Magnetic Resonance in Medicine. 2018;79(6):3207–3217.
[7] Suzuki Y, Nakamura Y, Yamada K, et al. Aquaporin-4 Positron Emission Tomography Imaging of the Human Brain: First Report. Journal of Neuroimaging. 2013;23(2):219–223.
[8] Harrison I F, Ismail O, Machhada A, et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain. 2020;143(8):2576–2593.
Figures
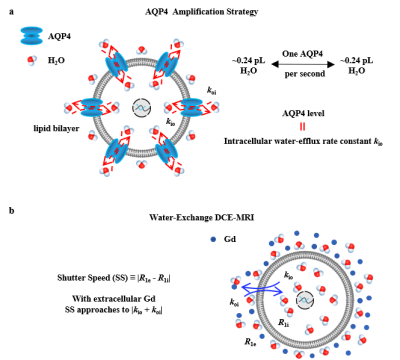
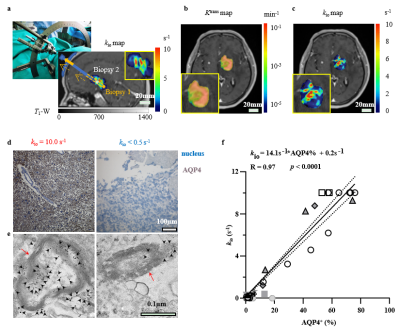
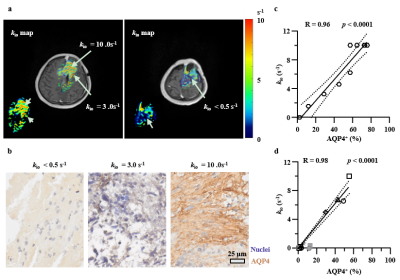
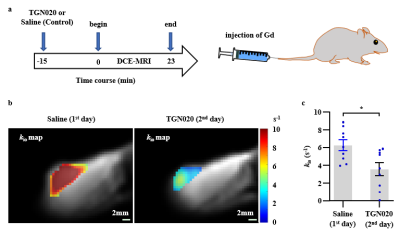
Figure 4. Effect of pharmacological inhibition of AQP4 on kio in the rat glioma. a: Timeline illustrating experiments used to quantify the effect of AQP4 inhibitor (TGN020) on kio in vivo. b: Effect of AQP4 inhibition on kio of glioma (C6) in the TGN020 group. c: The whole-tumor-averaged kio was decreased after the AQP4 inhibition with TGN020. Mean +/- standard error of the mean (SEM). paired t-test, * p<0.05.