0139
CEST Imaging of the APT and ssMT predict the overall survival of patients with glioma at the first follow-up after completion of radiotherapy at 3T1Division of Radiology, German Cancer Research Center, Heidelberg, Germany, 2Divion of Medical Physics in Radiology, German Cancer Research Center, Heidelberg, Germany, 3Department of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 4Division of Medical Physics in Radiology, German Cancer Research Center, Heidelberg, Germany, 5Department of Radiation Oncology, University Hospital Heidelberg, Heidelberg, Germany, 6Clinical Cooperation Unit Radiation Oncology, German Cancer Research Center, Heidelberg, Germany, 7Faculty of Medicine, University of Heidelberg, Heidelberg, Germany, 8Department of Neuroradiology, University Hospital Bonn, Bonn, Germany
Synopsis
Keywords: CEST & MT, Treatment, Glioma
In this prospective clinical study we compared the ability of asymmetry-based amide proton transfer-weighted (APTw) imaging with Lorentzian-fit-based (PeakAreaAPT and MTconst) and relaxation-compensated (MTRRexAPT and MTRRexMT) CEST-MRI of the amide proton transfer (APT) and semisolid magnetization transfer (ssMT) at 3T for the prediction of the overall survival of patients with glioma at the first follow-up after completion of radiotherapy. The APTw (HR=4.66, p<0.001) was more strongly associated with survival compared to the MTRRexAPT (HR=2.44, p=0.056) and MTconst (HR=2.54, p=0.044). The MTRRexMT and PeakAreaAPT did not display any association with survival.Introduction:
Differentiation of disease progression and radiation-induced changes is a major challenge in the clinical care for patients with diffuse glioma with possible implications for patient outcome[1]. Several groups have demonstrated that chemical exchange saturation transfer (CEST) MRI of the amid proton transfer (APT) and semisolid magnetization transfer (ssMT) is associated with therapy response and clinical outcome before radiotherapy[2-5]. Others have demonstrated that imaging of the APT can distinguish between disease progression and radiation-induced changes as early as 3 months following completion of radiotherapy[6-8]. However, there is a scarcity of cross-sectional trials comparing the clinical value of different CEST contrasts early after completion of radiotherapy in patients with glioma. In a prior study we have found that asymmetry-based APT-weighted (APTw) and Lorentzian-fit-based (PeakAreaAPT and MTconst) CEST imaging of the APT and ssMT could predict progression-free survival (PFS) in the first follow-up (in press). However, disease progression following radiotherapy is assessed by evaluation of volumetric changes of pathologic-appearing tissue on longitudinal MRI according to response assessment in neuro-oncology (RANO) criteria. This represents a well-known weakness of studies using PFS as reference standard. For this reason, we prospectively assessed the association of the APTw, PeakAreaAPT, MTconst and relaxation-compensated CEST contrasts of the APT and ssMT (MTRRexAPT and MTRRexMT) with the overall survival of patients with glioma in the first follow-up after completion of radiotherapy at 3T.Methods:
From July 2018 to December 2021, 54 study participants underwent CEST-MRI at 3T (Prisma®, Siemens) 4 to 6 weeks after completion of radiotherapy. Imaging of the APTw was performed according to Zhou J, et al., with four rectangular B1 pulses of 2μT[9]. The PeakAreaAPT, MTconst, MTRRexAPT and MTRRexMT were acquired with two low-power B1 pulses of 0.6 and 0.9 μT according to Mehrabian et al. [2] and Goerke et al.[10]. 3D tumor segmentations of glioma-associated contrast enhancement (CE) and whole tumor (WT) were performed on contrast-enhanced T1w (T1w-CE) and T2w-TIRM (T2w) images in Matlab® (Mathworks). Association of mean contrast values with overall survival (OS) was assessed by Kaplan-Meier curves and logrank-tests in all study participants (sub-cohort 1, n=54), in participants without midline gliomas (subcohort 2, n=49) and in participants without midline gliomas with residual CE (subcohort 3, n=34). Statistical testing was performed in Matlab®.Results:
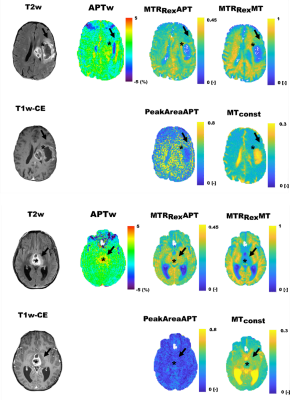
Exemple contrast maps of a study participant with gliobastoma and a participant with midline glioma are shown in Figure 1.Median OS for all participants was 12.3 months (min. 2.1 and max. 42.2 months) with 32/54 participants having reached an endpoint at data cut-off on May 3rd 2022.
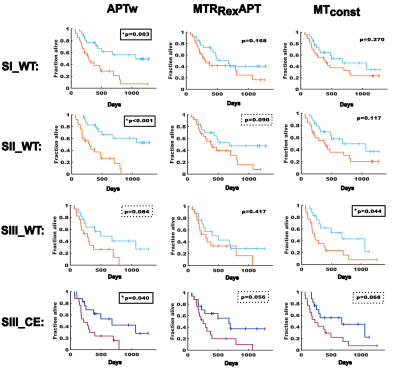
The APTw demonstrated the strongest association with OS. In sub-cohort 1 OS was 271 days vs. 434 days for participants with mean contrast values above and below median respectively for WT (HR=3.19, p=0.003). In sub-cohort 2 OS was 225 vs. 514 days for WT (HR=4.66, p<0.001). In sub-cohort 3 OS was 215 vs. 392 days for CE (HR=2.63, p=0.04) and 225 vs 398 days for WT (HR=2.3, p=0.08).
The MTconst demonstrated an association of mean contrast values with OS in sub-cohort 3 for WT (215 vs. 392 days, HR=2.54, p=0.04) and a tendency for CE (228 vs. 315 days, HR=2.33, p=0.07).
The MTRRexAPT showed a trend towards shorter survival of participants with higher mean contrast values for WT in sub-cohort 2 (372 vs. 458 days, HR=2.03, p=0.09) and CE in sub-cohort 3 (225 vs. 416 days, HR=2.44, p=0.056), but just missed significance.
The PeakAreaAPT and MTconst were not associated with survival. Kaplan-Meier plots for the APTw, MTRRexAPT and MTconst are displayed in Figure 2.
Discussion:
In this prospective clinical trial in a cohort of 54 study participants with glioma, we observed an association of the APTw and MTconst with OS with superior performance of the APTw. For the MTRRexAPT we also observed a trend towards shorter survival of participants with higher mean contrast values that just missed level of significance. In a prior study (in press) we observed an association of the APTw, PeakAreaAPT and MTconst with PFS in a very similar clinical cohort with superior performance of the MTconst. The superior performance of the APTw and the improving performance of the MTRRexAPT in the current study might indicate that OS could be a better response parameter for the assessment of true glioma progression in studies investigating the clinical value of CEST contrasts, compared to observational end points based on RANO criteria. The MTconst and PeakAreaAPT have substantial contributions from T1 and MT effects. This might help to explain the strong association of the MTconst and PeakAreaAPT with PFS, which might be related to a greater impact of therapy-induced changes. However, regardless of OS or PFS being used as the primary response parameter, the APTw was the only contrast that was associated with survival if no restriction was made for glioma location. This might indicate that CEST contrasts of glioma tissue could be influenced by adjacent brain structures.Conclusion:
The APTw is more strongly associated with the overall survival of patients with glioma in the first follow-up after completion of radiotherapy compared to Lorentzian-fit-based and relaxation-compensated CEST contrasts of the APT and ssMT at 3T.Acknowledgements
No acknowledgement found.References
1. Zikou, A., et al., Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol Imaging, 2018. 2018: p. 6828396.
2. Mehrabian, H., et al., Evaluation of Glioblastoma Response to Therapy With Chemical Exchange Saturation Transfer. Int J Radiat Oncol Biol Phys, 2018. 101(3): p. 713-723.
3. Paech, D., et al., Relaxation-compensated amide proton transfer (APT) MRI signal intensity is associated with survival and progression in high-grade glioma patients. Eur Radiol, 2019. 29(9): p. 4957-4967.
4. Regnery, S., et al., Chemical exchange saturation transfer MRI serves as predictor of early progression in glioblastoma patients. Oncotarget, 2018. 9(47): p. 28772-28783.
5. Chan, R.W., et al., Quantitative CEST and MT at 1.5T for monitoring treatment response in glioblastoma: early and late tumor progression during chemoradiation. J Neurooncol, 2021. 151(2): p. 267-278.
6. Ma, B., et al., Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J Magn Reson Imaging, 2016. 44(2): p. 456-62.
7. Park, K.J., et al., Added value of amide proton transfer imaging to conventional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur Radiol, 2016. 26(12): p. 4390-4403.
8. Liu, J., et al., Diagnostic performance of multiparametric MRI in the evaluation of treatment response in glioma patients at 3T. J Magn Reson Imaging, 2020. 51(4): p. 1154-1161.
9. Zhou, J., et al., APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J Magn Reson Imaging, 2019. 50(2): p. 347-364.
10. Goerke, S., et al., Relaxation-compensated APT and rNOE CEST-MRI of human brain tumors at 3 T. Magn Reson Med, 2019. 82(2): p. 622-632.
Figures