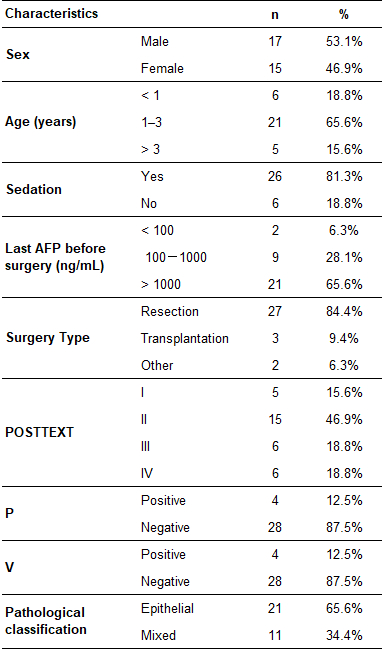
0137
Pediatric Hepatoblastoma After Neoadjuvant Chemotherapy: Diagnostic Performance of MR In Staging POSTTEXT and Vascular Involvement.
Li Jun Qian1, Xu Hua Gong1, Ming Xuan Feng2, Hai Nan Ren1, Yan Zhou1, Jian Rong Xu1, Qiang Xia2, and Yang Song3
1Radiology, Renji Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China, 2Liver Surgery, Renji Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China, 3MR Scientific Marketing, Siemens Healthineers Ltd., Shanghai, China
1Radiology, Renji Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China, 2Liver Surgery, Renji Hospital, Shanghai Jiao Tong University, School of Medicine, Shanghai, China, 3MR Scientific Marketing, Siemens Healthineers Ltd., Shanghai, China
Synopsis
Keywords: Cancer, Liver, Hepatoblastoma
This study evaluates the diagnostic efficacy of contrast-enhanced MR imaging for preoperative POSTTEXT staging and prediction of vascular involvement in pediatric hepatoblastoma after neoadjuvant chemotherapy. The findings revealed that MR preoperative POSTTEXT staging has a good level of agreement with the reference standard. MR provides high predictive performance for identifying portal vein involvement and moderate predictive performance for identifying hepatic vein/inferior vena cava involvement.Purpose
Hepatoblastoma is the most common pediatric liver malignant neoplasm, typically affecting patients aged 6 months to 3 years. Neoadjuvant chemotherapy is commonly used before surgery to improve the rate of complete resection of pediatric hepatoblastoma and reduce tumor recurrence, and the assessment of resectability after chemotherapy is highly dependent on POSTTEXT staging and its annotation factors P (portal vein involvement) and V (hepatic vein or inferior vena cava [IVC] involvement)1,2. Recently, free-breathing radial stack-of-stars 3D Dixon gradient echo sequence (StarVibe) has enabled the acquisition of high-quality contrast-enhanced MR images in pediatric patients3. It has not yet been documented if MR can be utilized as an accurate preoperative evaluation technique. We aim to assess the diagnostic performance of contrast-enhanced MR imaging for preoperative POSTTEXT staging and prediction vascular involvement in terms of annotation factors P and V in pediatric hepatoblastoma after neoadjuvant chemotherapy.Methods
This study was approved by the institutional review board with informed consent waived. From September 2021 to September 2022, a total of 32 consecutive pathologically proven pediatric hepatoblastoma patients who had MRI following neoadjuvant chemotherapy with a time interval between MRI and surgery of fewer than 14 days were collected retrospectively (Fig. 1). MRI was performed on a 3T scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany), with coronal T2w Half-Fourier acquisition single-shot turbo spin-echo (HASTE), axial T2w HASTE fat-suppression, axial DWI. For dynamic imaging, axial T1w StarVibe Dixon (Trajectory = Radial, FOV = 300 mm × 300 mm, TR/TE = 2.65/1.42 ms, NSA = 1, Flip angle = 9 deg, GRAPPA = None, Bandwidth = 980 Hz/px, Radial views = 384, voxel size = 1.2 mm × 1.2 mm × 2.5 mm) was performed before and 0 s, 49 s, and 137 s after contrast administration of 0.1 mmol of gadopentetate gadobutrol (Gadavist, Bayer Healthcare) per kilogram of body weight at a rate of 1 mL/s with saline flush. Coronal Cartesian T1w Vibe Dixon (Trajectory = Cartesian, FOV = 280 mm × 350 mm, TR/TE/TI = 4.22/1.32/2.55 ms, NSA = 6, Flip angle = 9 deg, GRAPPA = CAIPIRINHA:2, Bandwidth = 980 and 750 Hz/px, voxel size = 0.6 mm × 0.6 mm × 2.5 mm, Radial views = None) was performed 98 s after contrast. The entire protocol takes about 20 minutes from the localizers to the end of the scan. Two radiologists jointly reviewed the MR images and recorded POSTTEXT stages as well as annotation factors P, V, E (extrahepatic disease contiguous with the main liver tumor), C (caudate involvement), F (multifocality), N (lymph node metastases), R (tumor rupture), M (distant metastases) based on 2017 PRE/POSTTEXT system. The combination of operational records and pathologic findings served as the reference standard. Preoperative MR POSTTEXT staging was compared with the reference standard using the Kappa test. Diagnostic performances of MR in assessing P and V status were evaluated using the receiver operating characteristic (ROC) curve. The discrepancies between MR POSTTEXT and reference standard were further analyzed.Results
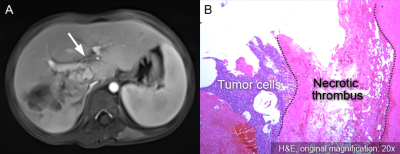
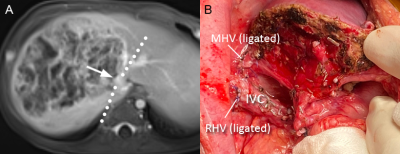
Among 32 patients, 5 (15.6%) were POSTTEXT stage I, 15 (46.9%) were stage II, 6 (18.8%) were stage III, 6 (18.8%) were stage IV. Preoperative MR POSTTEXT staging was correct in 20 patients (62.5%, weighted Kappa 0.61; 95% CI, 0.42 to 0.80). Four patients had portal vein involvement and four had hepatic vein/IVC involvement (Fig. 2 and 3). The sensitivity, specificity, and AUC were 100%, 96.4%, and 0.982 for portal vein involvement and 75.0%, 75.0%, and 0.750 for venous/IVC involvement, respectively. Overstaging was present in four (12.5%) patients (two cases overstaged I to II, and two cases II to III), and understaged in eight (25.0%) patients (two cases understaged II to I, three III to II, and three IV to III). The reasons for these 12 mis-staging cases including difficulty in determining the relationship between lesion boundaries and the landmark vessels (n = 4) (Fig. 4), discrepancy of caudate lobe boundary between radiologist and surgeon (n = 3) (Fig. 5)4, microlesions (n = 2), pedunculated tumor (n = 1), large tumor bulging landmark vessels (n = 1), and duplicated left hepatic vein (n = 1).Conclusion
A 20-minute free-breathing MRI contrast-enhanced examination for preoperative POSTTEXT assessment is feasible in pediatric hepatoblastoma patients. Preoperative MR POSTTEXT staging has a good level of agreement with surgical and pathological findings. MR has excellent accuracy for assessing P-status and moderate accuracy for V-status. The non-excellence performance of MR in assessing V status is most likely due to StarVibe's inadequate resolution in the z-axis (2.5mm), through which the hepatic veins and inferior vena cava travel. As StarVibe does not yet support coronal scans, coronal contrast-enhanced images using Cartesian trajectory remain susceptible to motion artifacts.Acknowledgements
No acknowledgement found.References
- Zhou S, Malvar J, Chi YY, et al. Independent Assessment of the Children’s Hepatic Tumors International Collaboration Risk Stratification for Hepatoblastoma and the Association of Tumor Histological Characteristics With Prognosis. JAMA Netw Open. 2022;5(2):e2148013.
- Towbin AJ, Meyers RL, Woodley H, et al. 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol. 2018;48(4):536-554.
- Duffy PB, Stemmer A, Callahan MJ, et al. Free-breathing radial stack-of-stars three-dimensional Dixon gradient echo sequence in abdominal magnetic resonance imaging in sedated pediatric patients. Pediatr Radiol. 2021;51(9):1645-1653.
- Kumon M. Anatomical Study of the Caudate Lobe with Special Reference to Portal Venous and Biliary Branches Using Corrosion Liver Casts and Clinical Application. Liver Cancer. 2017;6(2):161-170.
Figures
Patient demographics
Portal vein involvement in a four-year-old girl with
hepatoblastoma in the right and caudate lobes. (A) Portal venous phase MR image
shows neoplastic thrombus (arrow) in the left portal vein (P+). (B)
Photomicrograph (original magnification, 40×; hematoxylin-eosin [H-E] stain) shows
necrotic neoplastic thrombus adjacent to tumor cells.
Misinterpreted IVC involvement in a six-month-old girl with
hepatoblastoma in the right lobe. (A) Venous phase contrast-enhanced MR image
shows tumor encases intrahepatic IVC with more than 180° (V+). (B) The intrahepatic
IVC was found being pushed rather than invaded by the tumor, and the tumor was
completely removed. The intraoperative photograph shows the intact intrahepatic
IVC with ligated right and middle hepatic veins.
A 26-month-old boy with hepatoblastoma in segments 4 and
5 (POSTTEXT III), which was underestaged as POSTTEXT II at preoperative MR
study. (A) Non-contrast MR image shows a small portion of the tumor extending
from segments 4 to 5, which is hardly discernible on (B) contrast-enhanced MR image.
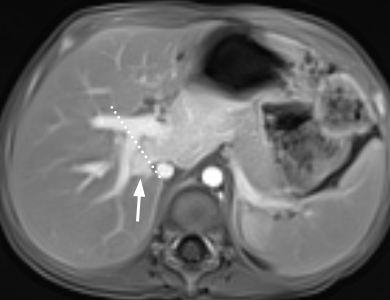
Discrepancy between
radiologist and surgeon on caudate lobe boundary in a 27-month-old boy with
hepatoblastoma. Some hepatic surgeons are used to further subdivide the caudate
lobe into the Spiegel lobe, paracaval section, and caudate process. The right
boundary of the caudate lobe could be more right-sided compared to that defined
by radiologist (dotted line). The tumor (arrow) was described in the operation
record as being confined in segments 1 and 4 (POSTTEXT II) but considered to
involve segments 1, 4, and 7 by radiologists (POSTTEXT III).
DOI: https://doi.org/10.58530/2023/0137