0131
Feasibility of oxygen-enhanced (OE) and intravoxel incoherent motion (IVIM) MRI for detection of HN cancer radiation therapy induced changes1Department of Medical Radiation Sciences, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 2Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden, 3Department of Oncology and Radiotherapy, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Synopsis
Keywords: Multi-Contrast, Radiotherapy, Diffusion/Other Diffusion Imaging Techniques
Tumor oxygenation is a biomarker proposed as a predictor of radiation therapy (RT) response. Here, the feasibility of Oxygen-Enhanced MRI, intravoxel incoherent motion, and diffusion kurtosis imaging for monitoring of oxygenation changes in head and neck cancers was evaluated. Seven patients were examined pre- and mid-RT. No relative change in population mean longitudinal relaxation rate was observed following RT. A general increase was noticed in population mean diffusion and capillary perfusion fraction, and a decrease in population mean kurtosis. The implementation of these techniques was clinically feasible, and relative changes in almost every derived biomarker could be observed following RT.Purpose
Relative changes of MRI derived biomarkers for tumor oxygenation during treatment might be predictive for radiation therapy (RT) response and clinical outcome1,2, and hence act as a noninvasive tool for personalized RT. The aim of this study was to evaluate the feasibility of Oxygen-Enhance MRI (OE-MRI), intravoxel incoherent motion (IVIM), and diffusion kurtosis imaging (DKI) for early response assessment in head and neck (HN) cancers.Methods
For seven HN cancer patients, morphological (T2W DIXON TSE and contrast enhanced T1W Dixon VIBE) and functional (OE-MRI, IVIM/DKI, and dynamic contrast enhanced (DCE)) MRI were acquired before and approximately two weeks after start of RT, using a RT equipped 1.5 T Siemens Aera wide bore MR-system (Siemens Healthcare, Erlangen, Germany) and a 20-channel head coil.OE-MRI was performed by acquisition of five dynamic MP2RAGE scans (i.e., dynamic TOLD series) with breathing of 100% O2 during dynamic 2-4 and dynamic 5 acquired approximately 22 minutes after the end of O2 breathing. T1-maps were created from the dynamic TOLD series data by simulations of the Bloch equations for the MP2RAGE sequence, for calculation of change in longitudinal MRI relaxation rate (ΔR1) (Fig 1).
Mean ΔR1 of each voxel for dynamic 3 – 4 was calculated, creating a modified TOLD (MTOLD) data set. The MTOLD-data was used to calculate mean ΔR1 within the tumor volume at both pre- and mid-RT, and relative changes evaluated. The DCE-data was used to classify voxels as perfused or non-perfused, following classifying the perfused tumor voxels either as oxygen-enhancing (Oxy-E) or oxygen-refractory (Oxy-R). A perfused tumor voxel was classified as Oxy-E if the corresponding voxel in MTOLD was larger than 2∙R1,dyn1∙CoV (coefficient of variation), where CoV was estimated using T1-values in the cerebellum from the dynamic TOLD series. All remaining perfused voxels were classified as Oxy-R. Hence, for all cases with DCE-data available, all voxels within the tumor volume were classified either as perfused Oxy-E (normoxia), perfused Oxy-R (hypoxia), or non-perfused (necrosis).
IVIM/DKI measurements was performed by acquisition of a single shot echo planar imaging sequence with four b-values (b = 0, 110, 650 and 1500 s/mm2, number of averages = 1, 2, 3, 2) and six diffusion weighting directions. The IVIM diffusion coefficient (D), capillary perfusion fraction (f), and kurtosis (K) effects were evaluated by segmented fitting of the data to the IVIM/DKI signal representation. The mean values of IVIM-derived (D and f) and DKI-derived (K) parameters were calculated within the tumor volume at both pre- and mid-RT, and relative changes evaluated.
Results and Discussion
OE-MRI and IVIM/DKI imaging were clinically feasible during RT MR-simulation, with successfully acquired OE-MRI data for six out of seven study patients, and IVIM/DKI data for all seven study patients.For OE-MRI, an increasing change of ΔR1 during O2 breathing is anticipated in non-hypoxic tissue while a constant ΔR1 is expected in hypoxic tissue3. A decrease in the amount of tumor hypoxia, corresponding to an increased mean ΔR1 during the course of treatment, is assumed to be related to a positive RT treatment outcome4. No relative change in population mean ΔR1 (Fig 2) or in the fraction of hypoxic voxels for pre- and mid-RT tumors was found. For IVIM/DKI, an increase of D and f indicates an increase of the mobility of water and microvascular blood volume, and a decrease in K implies a progression towards reduced microstructural heterogeneity. A general increase in population means of D and f, and a decrease in population mean of K was noticed over the course of RT, although not statistically proven (Fig 3).
For one study patient, relative biomarker changes corresponding to a prediction of early locoregional control according to literature were observed (illustrated as parameter maps in Fig 2c and Fig 3d). As the treatment outcome for the study patients is still unknown, no interpretation of unsuccessful/successful tumor response could be established.
Conclusion
The implementation of OE-MRI and IVIM/DKI imaging are feasible in a clinical RT setting, and changes of the derived biomarkers could be monitored during the course of treatment. By introduction of parameter maps that have the potential to visualize regions of hypoxia within the tumor, RT planning of localized treatment intensification or de-escalation strategies and thereby adaptive individualized RT may be enabled. Larger study cohort and future work regarding correlations of relative biomarkers changes to the treatment outcome are required to conclude the final prediction value of OE-MRI and IVIM/DKI imaging for early response assessment.Acknowledgements
The authors would like to thank the clinical staff at the center for all their help throughout the study.References
1. Hill RP, Bristow RG, Fyles A, Koritzinsky M, Milosevic M, Wouters BG. Hypoxia and Predicting Radiation Response [published online ahead of print 2015/09/19]. Semin Radiat Oncol. 2015;25(4):260-272.
2. Martens RM, Koopman T, Lavini C, et al. Early Response Prediction of Multiparametric Functional MRI and (18)F-FDG-PET in Patients with Head and Neck Squamous Cell Carcinoma Treated with (Chemo)Radiation [published online ahead of print 2022/01/12]. Cancers (Basel). 2022;14(1).
3. O'Connor JPB, Robinson SP, Waterton JC. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI [published online ahead of print 2018/10/03]. Br J Radiol. 2019;92(1095):20180642.
4. Salem A, Little RA, Latif A, et al. Oxygen-enhanced MRI Is Feasible, Repeatable, and Detects Radiotherapy-induced Change in Hypoxia in Xenograft Models and in Patients with Non-small Cell Lung Cancer [published online ahead of print 2019/05/06]. Clin Cancer Res. 2019;25(13):3818-3829. 18/10/03]. Br J Radiol. 2019;92(1095):20180642.
Figures
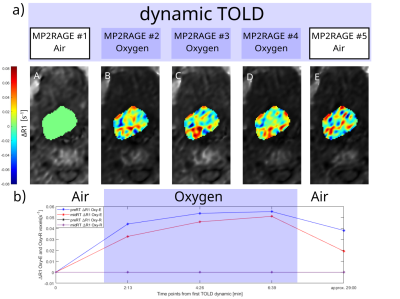
Fig 1. Illustration of OE-MRI for one study case: (a) Five dynamic MP2RAGE images and corresponding longitudinal ∆R1 parameter maps (A-E) normalized towards MP2RAGE #1. The ∆R1 parameter maps together with DCE data was used to classify perfused voxels as oxygen-enhancing (Oxy-E) or oxygen-refractory (Oxy-R). (b) The mean ∆R1 for Oxy-E classified voxels and Oxy-R classified voxels for each timepoint in the dynamic TOLD series at both pre- and mid-treatment MRI examination. As voxels were classified as Oxy-E while breathing 100% O2, this case shows successfully acquired OE-MRI data.
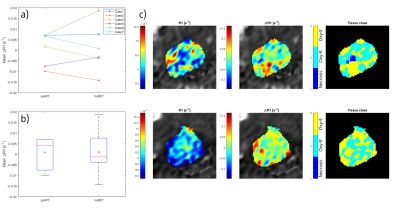
Fig 2. (a) Individual mean ΔR1 and (b) the mean ΔR1 for all study cases, within the tumor volume in the MTOLD at pre- and mid-radiation therapy (RT) MRI sessions, respectively. The x represent population mean ΔR1. (c) Pre-RT (upper row) and mid-RT (lower row) OE-MRI parameter maps for one study case with relative biomarker changes corresponding to a prediction of early RT respond according to literature. A general decrease in R1 of MP2RAGE #1 (left) and increase in ΔR1 in the MTOLD (middle), and necrosis classified voxels (dark blue) were non-existing in-between MRI sessions for this case.
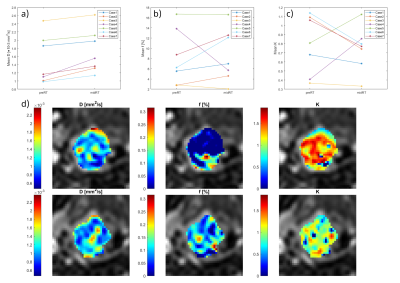
Fig 3. Individual mean (a) diffusion , (b) perfusion fraction, and (c) kurtosis, within tumor volumes from IVIM/DKI data at pre- and mid-radiation therapy (RT) MRI sessions for all study patients. (d) Pre-treatment (upper row) and mid-treatment (lower row) IVIM/DKI parameter maps for the same study case as in figure 2. The parameter maps presented are diffusion (left), perfusion fraction (middle), and kurtosis (right). A general increase in diffusion and perfusion fraction can be seen at the mid-treatment imaging session and a kurtosis decreased in-between the two imaging sessions.