0128
Acute tumour response to STING activation assessed with multi-parametric MRI1Radiotherapy and Imaging, The Institute of Cancer Research, Sutton, United Kingdom, 2Pharma GmbH & Co. KG, Boehringer Ingelheim, Biberach, Germany, 3RCV GmBH & Co KG, Boehringer Ingelheim, Vienna, Austria
Synopsis
Keywords: Cancer, Drug Development, Preclinical, Diffusion Imaging, Relaxometry
Whilst cancer immunotherapies have shown marked and durable tumour responses in some patients, the majority derive no benefit. Strategies are being exploited to enhance tumour responses to immuno-oncology agents, including pharmacological activation of the STING pathway to create a more inflamed microenvironment. Multi-parametric MRI revealed a dose-dependent increase in murine tumour ADC in response to a STING agonist that was associated with histologically confirmed increase in tumour cell death. An acute, transient increase in tumour R2*, consistent with vascular occlusion, was also found. ADC is a sensitive early imaging biomarker of tumour response to STING agonism.Introduction
Whilst cancer immunotherapies have shown marked and durable tumour responses in some patients, the majority don’t derive long term benefit. Various strategies are being exploited to enhance tumour responses to immuno-oncology agents. The cytoplasmic DNA-sensing cyclic GMP-AMP synthase (cGAS)-stimulator of interferon (IFN) genes (STING) pathway controls the transcription of numerous genes including type I IFNs and pro-inflammatory cytokines, driving T-cell priming, activation and infiltration into the tumour microenvironment1. Pharmacological activation of STING may augment tumour immunogenicity, creating a more inflamed microenvironment and enhanced response to immunotherapy and radiotherapy2.Quantitative multiparametric MRI can enable an understanding of the tumour microenvironment and inform on early treatment response3,4. Incorporation of multiparametric MRI for the pre-clinical evaluation of STING agonists could accelerate their clinical development through rapid assessment of successful tumour response and dose finding in vivo.
Here we demonstrate the utility of diffusion-weighted MRI and transverse MR relaxometry to assess tumour response to STING agonism.
Methods
Murine TBPt-4C4 thyroid cancer cells (2.5x106) were injected subcutaneously into the flanks of female C57BL/6J mice. MRI was performed when tumours reached ~150–200 mm3. Mice received a single intravenous dose of either vehicle alone (n=6), 7µmol/kg (n=8) or 20µmol/kg (n=8) STING agonist (Boehringer Ingelheim).MRI data were acquired prior to, and 4h and 24h after treatment from anaesthetised mice (2% isoflurane in air) on a Bruker 7T microimaging system using a 40mm volume coil. Following acquisition of multi-slice T2-weighted RARE (TR/TE=4.5s/36ms) images for tumour localisation and volume determination, diffusion-weighted spin echo (five b-values: 200-1000s/mm2), multiple gradient echo (TR/TEs=200/3-24ms, 8 echoes) and inversion recovery true-FISP (TR/TE=3.4/1.7ms, 50 TIs: 72.1-2738ms) images were acquired from a single central 1mm slice using a 128x128 matrix over a 3cm FOV.
Parametric maps of ADC, R2* and T2 were calculated voxel wise using in-house software (ImageView, written in IDL), and median values calculated from a region-of-interest encompassing the whole tumour5. Maps of R2´ were assessed assuming R2´=R2*-R2 (=1/T2). Significant changes in the MRI parameters were identified using Student’s paired t-test.
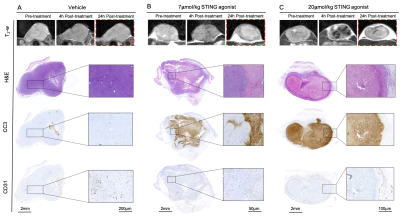
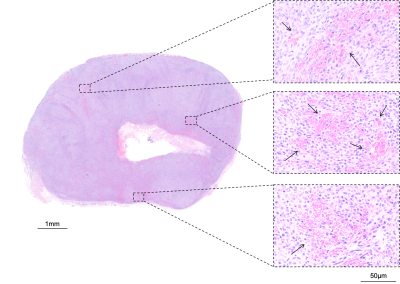
Following the final scan, tumours were carefully excised and MRI-aligned FFPE sections were either tinctorially stained with haematoxylin and eosin (H&E) or immunohistochemically processed for cleaved caspase 3 (CC3) and CD31 for detection of apoptosis or vascular density, respectively.
Results
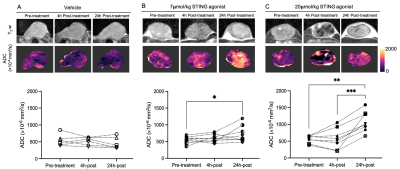
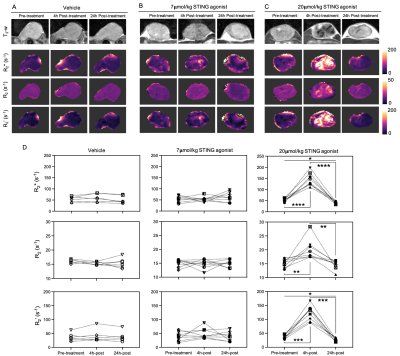
Whilst the T2-weighted signal intensity of images from control and pre-treatment tumours was relatively homogeneous, areas of both hypo- and hyperintensity were apparent in the STING agonist challenged tumours, particularly at 24h post-treatment (Figure 1). A temporal increase in water diffusivity was observed in the low-dose cohort, whereas for some of the high-dose-treated mice a small reduction in ADC was seen at 4h followed by a clear increase at 24h. Tumour ADC significantly increased 24h after treatment with 7 and 20µmol/kg STING agonist, suggesting dose dependency.There was no significant change in R2* in the vehicle and low-dose-treated cohorts (Figure 2). However, treatment with 20µmol/kg induced a dramatic and highly significant increase in R2* across the whole tumour 4h post-treatment, followed by a clear and significant reversion to baseline levels at 24h. Assessment of the relative contributions of the irreversible (R2) and reversible (R2´) transverse relaxation rates revealed that the temporal changes seen in R2* were dominated by changes seen in R2´.
H&E and CC3-stained tumour sections showed that STING agonist induced tumour cell death 24h after treatment in an apparent dose-dependent manner (Figure 3). 20µmol/kg STING agonist caused massive central tumour cell death surrounded by a viable rim. CD31 staining demonstrated a dose-dependent reduction in vascular density within viable tumour regions.
Discussion
The acute and transient reduction in ADC seen 4h after treatment with 7µmol/kg STING agonist is consistent with initial cell swelling and consequent impeded water diffusion in the extravascular space6. The subsequent increase in ADC observed at 24h post-treatment with 7 or 20µmol/kg STING agonist was spatially associated with histologically confirmed tumour cell death. The resultant loss of cell membrane integrity, reduced cell density and increased interstitial space enables increased water diffusion and, hence, ADC at this timepoint4, 6.The acute increase in tumour R2* seen at 4h post-treatment with 20µmol/kg STING agonist is consistent with the aggregation of deoxygenated, and hence paramagnetic, erythrocytes associated with an ischaemic insult (Figure 4). A similar increase in tumour R2* has been observed in response to the murine-specific STING agonist DMXAA, and other vascular disrupting agents7, 8.
The subsequent recovery of R2* at 24h was associated with extensive cell death and negligible vascularity. One explanation is clearance of erythrocytes by macrophages. Another is an increase in the water/macromolecule ratio caused by oedema, and accordingly a significant reduction in tumour R2 was found. However, this only constituted ~20% of the reduction in R2* compared to pre-treatment value, which was clearly dominated by a reduction in R2´. Calculated maps of R2´, which are highly sensitive to magnetic field inhomogeneities, suggest the absence of any paramagnetic species at this timepoint, and may reflect denaturation of paramagnetic deoxyhaemoglobin to diamagnetic products such as hemichromes7.
Conclusion
Multi-parametric MRI identified increased ADC as a sensitive early imaging biomarker of tumour response to STING agonist. Quantitation of tumour R2* may provide a complementary exploratory imaging biomarker of acute vascular response to STING agonism.Acknowledgements
We acknowledge support from Cancer Research UK grant C16412/A27725 and The Barrow Neurological Foundation.References
1. Jing W, et al. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. Journal for ImmunoTherapy of Cancer 2019;7:115.
2. McLaughlin M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer 2020;20:203-217.
3. O’Connor JPB, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 2017;14:169-186.
4. Sinkus R, et al. Apparent diffusion coefficient from magnetic resonance imaging as a biomarker in oncology drug development. Eur J Cancer 2012;48:425-431.
5. Walker-Samuel S, et al. Robust estimation of the apparent diffusion coefficient (ADC) in heterogeneous solid tumors. Magn Reson Med 2009;62:420-429.
6. Galbán CJ, et al. Diffusion MRI in early cancer therapeutic response assessment. NMR Biomed 2017;30:e3458.
7. Robinson SP, et al. Acute tumor response to ZD6126 assessed by intrinsic susceptibility magnetic resonance imaging. Neoplasia 2005:466-474.
8. McPhail LD, et al. Assessment of tumor response to the vascular disrupting agents 5,6-dimethylxanthenone-4-acetic acid or combretastatin-A4-phosphate by intrinsic susceptibility magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2007;69:1238-1245.
Figures