0127
Transformation of the histopathological growth pattern of colorectal liver metastases after chemotherapy predicted by an MRI radiomics model1Department of Radiology, Peking University People's Hospital, Beijing, China
Synopsis
Keywords: Radiomics, Machine Learning/Artificial Intelligence, Histopathological growth patterns; colorectal liver metastases; transformation
Our study used an MRI-based radiomics model to predict the transformation of the histopathological growth pattern (HGP) of colorectal liver metastases (CRLMs) before and after chemotherapy. After collecting data, drawing regions of interest and analyzing radiomics, we enrolled 152 patients and 299 liver metastases (99 pure desmoplastic (pdHGP) and 174 non-pdHGP). The pdHGP in the non-chemotherapy group and the post-chemotherapy group accounted for 28.3% and 42.5%, respectively (P=0.019). The fused MRI-based radiomics model demonstrated good predictive performance, and it could predict pdHGP before chemotherapy (25.3%), which was significantly different (p=0.034) compared with postoperative pdHGP after chemotherapy (39.1%).Abstracts
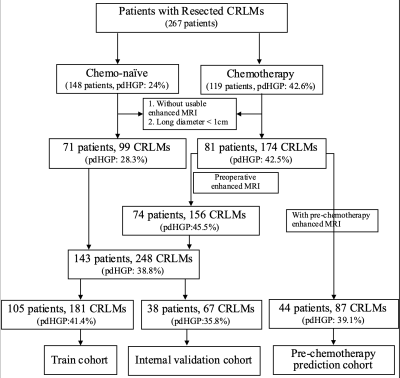
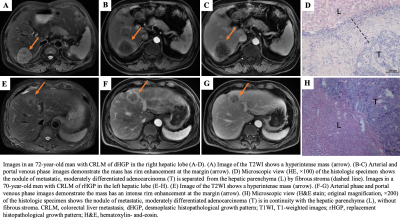
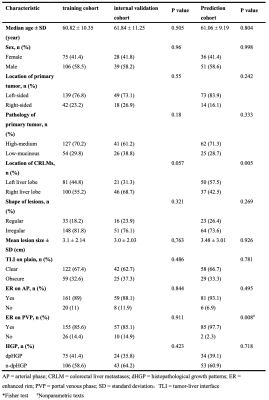
Introduction: Histopathological growth patterns (HGPs) are a reliable, reproducible and strong prognostic biomarker that can be assessed on hematoxylin and eosin-stained sections of surgically resected colorectal liver metastases (CRLMs). The update international consensus guidelines state that any proportion of non-desmoplastic HGP, no matter how small, is associated with a poorer prognosis1. Moreover, there are studies suggesting that chemotherapy induce the desmoplastic HGP in patients with replacement-type CRLM2, which could explain the survival benefit from pre-operative chemotherapy. However, it remains to be determined whether chemotherapy induced a transformation for the HGP, as a pathological diagnosis made from entire resect specimen, of the same liver metastatic tumor before and after chemotherapy cannot be obtained. Recently, several radiomics studies were used to predict the HGP of CRLMs3, 4, which makes it possible to define the type of HGP before the resection of CRLMs. Therefore, the aim of our study was to investigate whether the HGP of CRLMs could transit after chemotherapy by constructing an MRI-based radiomics model.Methods: Patients with histopathological confirmed CRLMs (long diameter > 1cm) were identified by MRI between January 2007 and July 2022. The HGPs of CRLMs were classified as either pure desmoplastic HGP (pdHGP) or non-pdHGP based on entire resection surgery, including chemo-naive and post-chemotherapy cohorts. Radiomics analysis was performed on the tumor-liver interface (TLI) zone on the T2-MRI, arterial phases (AP) and portal venous phases (PVP). Lesions were divided into training, internal validation datasets based on time. The Pearson test and least absolute shrinkage and selection operator (LASSO) algorithm were used for feature selection, and multivariable logistic regression analyses and receiver operating characteristic (ROC) curves were used to evaluate the model performance. Furthermore, we used this model to predict pre-chemotherapy HGP in patients undergoing neoadjuvant chemotherapy followed by resection.
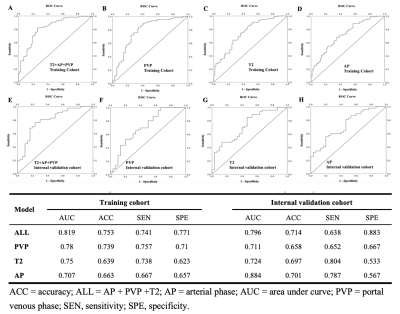
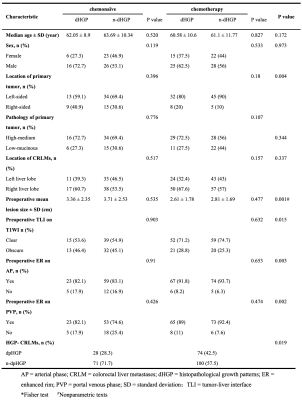
Results: Among 152 patients (chemo-naive of 71 and post-chemotherapy of 81 patients), there were 273 CRLMs (99 pdHGP and 174 non-pdHGP) were used to construct the predicting model. The prevalence of pdHGP was 28.3% and 42.5% in chemo-naive and post-chemotherapy, respectively (P=0.019). The radiomics signature consisted of 20 features of each phase selected. The AP+PVP+T2 fused radiomics signature demonstrated good predictive performance in distinguishing between pdHGP or non-pdHGPs (the AUC values were 0.819 and 0.798 in the training and internal validation group, respectively). By using the radiomic model, the HGP type could be seen for lesions before and after chemotherapy, with the pdHGP from 25.3% to 39.1% (p=0.034).
Discussion: In our study, we constructed a MR image based radiomics model which could predict pdHGP of CRLMs effectively. By comparing the baseline HGP predicted by this tool and the post-chemotherapy HGP diagnosed by resect specimen of a single cohort of CRLMs patients, we found that preoperative systemic chemotherapy could induce the non-pdHGP transit to the pdHGP (pdHGP prevalence from 25.3% to 39.1%). This result verified the hypothesis of HGP transformation in CRLMs after chemotherapy assessed by Hoppener et al5. In that pathological study, the conclusion of HGP transition was based on different cohorts for baseline HGP assessment was impossible. Furthermore, in our study, the proportion of pdHGP was 28.3% in the chemo-naive group and 42.5% in the post-chemotherapy group (P=0.019), which was similar to the findings of Hoppener’s study5. The phenomenon of HGP transformation could be explained by the immune activation caused by pre-operative chemotherapy6.Although there were several radiomics studies on HGP predicting7, 8 , this was the first MRI based radiomics model in pdHGP prediction. MR is increasingly used in the detection and follow-up of liver metastases because of its high resolution of soft tissue. Although it was difficult to judge the type of HGP with the naked eye, a multiparameter MRI-based radiomics model could be used as a non-invasion tool to preoperatively distinguish the HGP of CRLMs effectively.
Conclusions: Pure desmoplastic HGP could be more prevalent in post-chemotherapy CRLMs, which could be explained by HGP transformation testified by a radiomics HGP predicting model derived from MRI images.
Acknowledgements
I would like to thank Dr. Wang and Dr. Cheng for their guidance on the method and writing of the article, and thanks to Mr. Liu Tao for his support in image data transmission.References
1. Latacz E, Hoppener D, Bohlok A, et al. Histopathological growth patterns of liver metastasis: updated consensus guidelines for pattern scoring, perspectives and recent mechanistic insights. Br J Cancer. 2022;127: 988-1013.
2. Nierop PM, Hoppener DJ, Buisman FE, et al. Preoperative systemic chemotherapy alters the histopathological growth patterns of colorectal liver metastases. J Pathol Clin Res. 2022;8: 48-64.
3. Wei S, Han Y, Zeng H, et al. Radiomics diagnosed histopathological growth pattern in prediction of response and 1-year progression free survival for colorectal liver metastases patients treated with bevacizumab containing chemotherapy. Eur J Radiol. 2021;142: 109863.
4. Han Y, Chai F, Wei J, et al. Identification of Predominant Histopathological Growth Patterns of Colorectal Liver Metastasis by Multi-Habitat and Multi-Sequence Based Radiomics Analysis. Front Oncol. 2020;10: 1363
5. Hoppener DJ, Galjart B, Nierop PMH, et al. Histopathological Growth Patterns and Survival After Resection of Colorectal Liver Metastasis: An External Validation Study. JNCI Cancer Spectr. 2021;5.
6. Hoppener DJ, Nierop PMH, Hof J, et al. Enrichment of the tumour immune microenvironment in patients with desmoplastic colorectal liver metastasis. Br J Cancer. 2020;123: 196-206.
7. Cheng J, Qiu M, Zhang Y, et al. Enhanced Rim on MDCT of Colorectal Liver Metastases: Assessment of Ability to Predict Progression-Free Survival and Response to Bevacizumab-Based Chemotherapy. American Journal of Roentgenology. 2020;215: 1377-1383.
8. Starmans MPA, Buisman FE, Renckens M, et al. Distinguishing pure histopathological growth patterns of colorectal liver metastases on CT using deep learning and radiomics: a pilot study. Clin Exp Metastasis. 2021;38: 483-494.
Figures