0125
Deep Learning Radiomic Analysis of DCE-MRI Predicts Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer1Department of Radiology, Suining Central Hospital, Suining, China, 2Medical AI Lab, School of Biomedical Engineering, Health Science Center, Shenzhen University, Shenzhen, China, 3School of Life Science, South China Normal University, Guangzhou, China, 4The First Clinical Medical College, Guangdong Medical University, Zhanjiang, China, 5Department of Radiology, Dongguan People's Hospital, Dongguan, China, 6Department of Radiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
Synopsis
Keywords: Breast, Treatment, dynamic contrast-enhanced magnetic resonance imaging, pathological complete response, radiomics
Based on pre-treatment and early treatment dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and clinical characteristics, we established a pathological complete response (pCR) prediction model using a deep learning radiomic (DLR) method that achieved good performance in the training and validation cohorts. The model can help clinicians evaluate whether the patient can reach pCR after neoadjuvant chemotherapy (NAC) and can provide an effective diagnostic reference for accurate medical treatment of patients receiving NAC.Introduction
Neoadjuvant chemotherapy (NAC) for breast cancer is a systemic chemotherapy conducted before surgery or radiotherapy. Its main purpose is to decrease tumor load before surgery, reduce tumor stage, and convert inoperable tumors into operable ones. As a result, female patients with breast cancer who require total mastectomy may manage to preserve their breasts and improve their overall treatment outcomes and their quality of life [1]. Furthermore, studies have shown a strong association between pathological complete response (pCR), overall survival, and disease-free survival [2]. Recent studies have shown that radiomic features obtained using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) have good predictive ability for pCR after the completion of NAC in breast cancer [3], and Peng et al [4] have used deep learning radiomic (DLR) based on DCE-MRI to predict pCR to NAC in breast cancer, but the predictive power of the models was found to be unsatisfactory. In addition, models that comprehensively incorporate pre-treatment and early treatment DCE-MRI data, as well as clinical information, are almost non-existent. As the pre-treatment image features and clinical features are related to the characteristics of the primary tumor, the post-treatment images can reflect the tumor response to NAC drugs. Here, we hypothesized that improved performance could be achieved by constructing a model combining these three elements. As a result, we retrospectively included women with breast cancer who received NAC and obtained radiomic and deep learning semantic segmentation features based on pre-treatment and early treatment DCE-MRI data. We aimed to develop a model combined multi-period images information with clinical characteristics to predict pCR in female patients with breast cancer treated with NAC.Methods
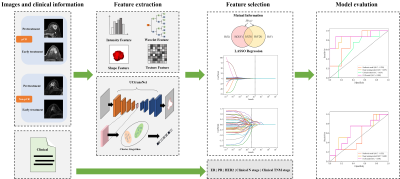
Ninety-five patients with breast cancer who underwent NAC and breast DCE-MRI from 2018 to 2021 were enrolled in the study. Several models were built to explore the performance differences of models constructed with various features over different periods. We connected the radiomic and deep learning features to construct the DLR model. To completely use all information from the traditional radiomic features, deep-learning semantic segmentation features, and clinical characteristics, we spliced these data to construct a combined model. The prediction results of the two models (constructed from the three features of pre-treatment and early treatment, respectively) were integrated. The output probability of the two models averaged the final probability of each patient. Finally, a logistic regression classifier was used to build our model from the selected best radiomic features, deep learning features, and clinical characteristics. Figure 1 shows an overview of our study.Results
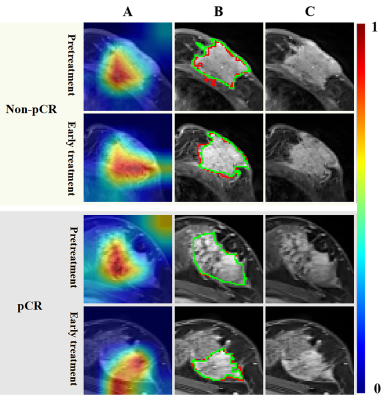
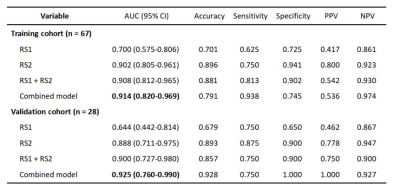
The performance of the DLR model combining pre- and early treatment information (AUC=0.900) was better than that of RS1 (AUC=0.644, P=0.068) and slightly higher that of RS2 (AUC=0.888, P=0.604) in the validation cohort. The combined model including pre- and early treatment information and clinical characteristics showed the best ability with an AUC of 0.925 in the validation cohort (Table 1). In this study, we visualized the deep learning features and segmentation results (Figure 2).Discussion
Radiomics has been successfully used to predict pCR after NAC in patients with breast cancer [5]. However, the AUC range of the pCR prediction model using the radiomic method based on pre-treatment DCE-MRI data was only 0.57–0.79 [6-7]. The AUC of the pCR prediction model using the radiomic method based on post-treatment DCE-MRI data was only 0.72 [8]. In addition, the DLR method has been successfully used in ultrasonography to predict the pCR of breast cancer to NAC [9]. However, to our knowledge, no DLR-related study has been conducted based on DCE-MRI data and clinical information obtained at multiple time points to predict pCR. We developed a combined model that integrates multi-period image features and clinical information, and the model in our study achieved the highest AUCs. Our results revealed that the performance of the model based on early treatment DCE-MRI data was better than that based on pre-treatment DCE-MRI data, proving the significant predictive value of early treatment DCE-MRI, which was consistent with that reported by Jiang et al [9]. The combination of clinical characteristics and imaging features allows for a more comprehensive description of breast tumors, and the performance of the model combining clinical characteristics with imaging features may be improved in some ways [10], which was confirmed by our study.Conclusion
We successfully constructed a deep learning-based radiomic model combined with clinical characteristics for predicting pCR to NAC in patients with breast cancer based on pre-treatment and early treatment DCE-MRI data.Acknowledgements
No acknowledgement found.References
1. You C, Zhou J, Tao K. Evaluation of neoadjuvant therapy for breast cancer based on radiomics. Chin J Radiol. 2021;55(11):1226-9. doi: https://doi.org/10.3760/cma.j.cn112149-20210912-008462.
2. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-72. doi: https://doi.org/10.1016/s0140-6736(13)62422-83.
3. Braman NM, Etesami M, Prasanna P, Dubchuk C, Gilmore H, Tiwari P, et al. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res. 2017;19(1):57. doi: https://doi.org/10.1186/s13058-017-0846-14.
4. Peng Y, Cheng Z, Gong C, Zheng C, Zhang X, Wu Z, et al. Pretreatment DCE-MRI-Based Deep Learning Outperforms Radiomics Analysis in Predicting Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Front Oncol. 2022;12:846775. doi: https://doi.org/10.3389/fonc.2022.8467755.
5. Liu Z, Li Z, Qu J, Zhang R, Zhou X, Li L, et al. Radiomics of Multiparametric MRI for Pretreatment Prediction of Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Multicenter Study. Clin Cancer Res. 2019;25(12):3538-47. doi: https://doi.org/10.1158/1078-0432.Ccr-18-31906.
6. Fan M, Chen H, You C, Liu L, Gu Y, Peng W, et al. Radiomics of Tumor Heterogeneity in Longitudinal Dynamic Contrast-Enhanced Magnetic Resonance Imaging for Predicting Response to Neoadjuvant Chemotherapy in Breast Cancer. Front Mol Biosci. 2021;8:622219. doi: https://doi.org/10.3389/fmolb.2021.6222197.
7. Eun NL, Kang D, Son EJ, Park JS, Youk JH, Kim JA, et al. Texture Analysis with 3.0-T MRI for Association of Response to Neoadjuvant Chemotherapy in Breast Cancer. Radiology. 2020;294(1):31-41. doi: https://doi.org/10.1148/radiol.20191827188.
8. Sutton EJ, Onishi N, Fehr DA, Dashevsky BZ, Sadinski M, Pinker K, et al. A machine learning model that classifies breast cancer pathologic complete response on MRI post-neoadjuvant chemotherapy. Breast Cancer Res. 2020;22(1):57. doi: https://doi.org/10.1186/s13058-020-01291-w9.
9. Jiang M, Li CL, Luo XM, Chuan ZR, Lv WZ, Li X, et al. Ultrasound-based deep learning radiomics in the assessment of pathological complete response to neoadjuvant chemotherapy in locally advanced breast cancer. Eur J Cancer. 2021;147:95-105. doi: https://doi.org/10.1016/j.ejca.2021.01.02810.
10. Xie Y, Zhang J, Xia Y, Fulham M, Zhang Y. Fusing texture, shape and deep model-learned information at decision level for automated classification of lung nodules on chest CT. Information Fusion. 2018;42:102-10. doi: https://doi.org/https://doi.org/10.1016/j.inffus.2017.10.005
Figures