0122
Accelerated single UTE-Dixon for simultaneous short T2*water and fat imaging using a FLORET trajectory1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Philips GmbH Market DACH, Hamburg, Germany, 3Philips Healthcare, Rochester, MN, United States, 4Department of Radiology, Mayo Clinic College of Medicine and Science, Rochester, MN, United States
Synopsis
Keywords: Bone, Skeletal
Ultra-short echo time (UTE) imaging enables the depiction of short-T2* tissues and is being increasingly used for the generation of CT-like bone images. UTE imaging has been recently combined with single-echo Dixon processing to enable the separation of water and fat signals from a single echo UTE image, but was primarily previously employed in radial stack-of-stars UTE acquisitions with prolonged scan durations. The present work combines single UTE-Dixon processing with a Fermat looped, orthogonally encoded trajectory (FLORET) to enable accelerated simultaneous short T2* water- and fat-separated imaging at sub-millimeter isotropic resolution. The technique is applied in the ankle.Purpose
Ultra-short echo time (UTE) imaging enables the generation of CT-like images to assess pathological bone changes1. Fat-suppression is important for UTE imaging to better depict the short T2* components of the musculoskeletal anatomy2-4. To this end Dixon imaging has been combined with UTE as an effective way to perform fat suppression with the typical requirement to acquire more than one echo5,6. A recently proposed single UTE-Dixon (sUTE Dixon) methodology enabled the simultaneous imaging of short T2* water and fat signals from a single complex UTE image, by removing unwanted low frequency background phases7. sUTE Dixon imaging has been applied for the simultaneous detection and assessment of acute vertebral fractures as well as bone marrow edema of the thoracolumbar spine7,8 and has also been shown to be feasible across various musculoskeletal anatomies9. However, sUTE Dixon imaging has been combined primarily with a radial stack-of-stars (SoS) readout trajectory, which is typically associated with prolonged acquisition times and therefore anisotropic resolutions. The present work proposes the combination of sUTE-Dixon processing with a Fermat looped, orthogonally encoded trajectory (FLORET) to accelerate sUTE-Dixon for simultaneous CT-like and short T2* water and fat imaging with high isotropic 3D resolution. Results of FLORET sUTE Dixon in the ankle with isotropic sub-millimeter resolution are presented.Methods
Measurements: The ankle of two volunteers were scanned in a 3 T scanner (Ingenia Elition X, Philips Healthcare) using a 16-channel foot/ankle coil. The previously proposed FLORET trajectory with Fibonacci interleaf ordering was employed with three hubs and $$${\alpha}_0 = 36^o$$$10,11. A 3D field-of-view of 120x120x120 mm3 was encoded with a 0.45x0.45x0.45 mm3 voxel size requiring a total number of 30804 spiral arms. The spiral readout duration was 2.48 ms. The employed flip angle was 8o and TE/TR = 0.17/9.5 ms. The total acquisition time for the FLORET UTE was 4 minutes 53 seconds. Two volunteers were scanned with the described FLORET UTE sequence. For comparison, one volunteer was also scanned with a SoS UTE sequence (FOV = 200x200x120 mm3, voxel size = 0.45x0.45x3 mm3, flip angle = 5o, TE/TR = 0.17/6.7 ms, scan time = 4 minutes 17 seconds).Processing: UTE images contain unwanted phase components $$$\phi(t)$$$ which arise due to B0 inhomogeneities, eddy currents, signal delays in the receiver chains, and B1 transmit/receive phase. Water and fat-separated images were obtained by solving the following smoothness-constrained non-linear inverse water-fat problem for the unwanted phase term $$$\phi$$$7:
$$\phi^* = \underset{\phi}{\textrm{argmin}}\frac{1}{2}\left \| \left ( W + Fe^{i\theta(\textrm{TE})} \right )e^{i\phi} -S_{exp}(\textrm{TE}) \right \|_2^2 + \lambda\left \| M\bigtriangledown \phi \right \|_2^2$$
where $$$\phi^*$$$: optimal solution which contains all unwanted phase components, W: magnitude of the water signal, F: magnitude of the fat signal, $$$\theta$$$(TE): phase difference between water and fat due to the chemical shift, Sexp: measured signal, $$$\lambda$$$: regularization parameter, M: mask which was derived from the magnitude images and $$$\bigtriangledown$$$ is the 3D gradient in the coordinate system of the acquired image.
Results
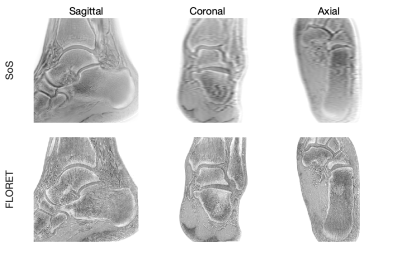
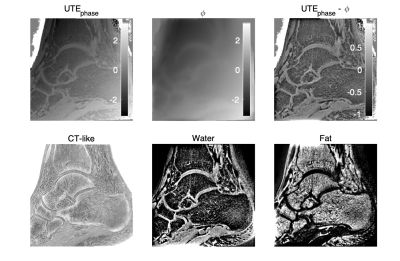
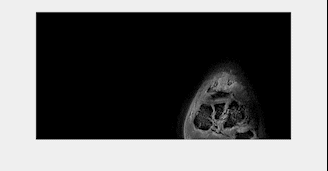
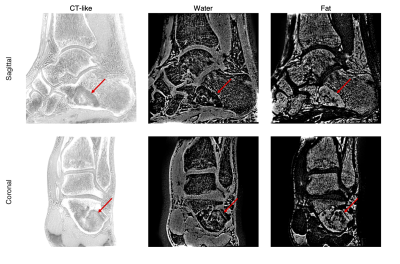
The inverted magnitude images of an ankle in three orthogonal planes from the SoS UTE and the FLORET UTE acquisitions were compared in Figure 1. Cortical and trabecular bone structures were well-depicted in the in-plane (sagittal) inverted magnitude (CT-like) images for both SoS and FLORET. In the reformatted coronal and axial planes, FLORET was superior due to its 3D isotropic voxel size. The phase of the FLORET UTE image shows unwanted low-resolution contamination (Figure 2). Solving Equation (1) with the regularization parameter removed the contamination phase and yielded high-quality water and fat separated images (Figure 2). The 3D isotropic voxel size of the FLORET UTE also allowed the visualization of part of the vascular tree within the ankle as shown in Figure 3. The second subject had an intraosseous lipoma within the calcaneus of the left ankle. The borders of the lipoma could be clearly delineated in the fat-separated images (Figure 4) and the central ossifications/calcifications (cockade sign) within the lipoma could be depicted in the inverted magnitude images (Figure 4).Discussion and Conclusion
High-resolution UTE imaging typically requires the use of efficient k-space trajectories. The FLORET trajectory was presently employed for high-isotropic resolution CT-like-imaging of short T2* species. The linear characteristic of the unwanted phase terms in FLORET UTE might indicate to be caused by gradient delays. However, the sUTE Dixon processing was able to remove these unwanted phase contributions, allowing to additionally reconstruct high quality water- and fat-separated images from the FLORET UTE data. The proposed combination of the FLORET UTE trajectory with the sUTE Dixon processing enables the simultaneous CT-like imaging of cortical bone with short T2* water-separated imaging of the vasculature and with short T2* fat-separated imaging of trabecularized bone marrow at sub-millimeter isotropic resolution in the ankle in clinically acceptable scan times.Acknowledgements
The present work was supported by Philips Healthcare and the German Research Foundation (project number 455422993, FOR5298-iMAGO-P1)References
1. Afsahi AM, Ma Y, Jang H, Jerban S, Chung CB, Chang EY, Du J. Ultrashort Echo Time Magnetic Resonance Imaging Techniques: Met and Unmet Needs in Musculoskeletal Imaging. J Magn Reson Imaging 2022;55(6):1597-1612.
2. Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magn Reson Med 2016;76(2):577-582.
3. Jang H, Carl M, Ma Y, Jerban S, Guo T, Zhao W, Chang EY, Du J. Fat suppression for ultrashort echo time imaging using a single-point Dixon method. NMR Biomed 2019;32(5):e4069.
4. Ma J. A single-point Dixon technique for fat-suppressed fast 3D gradient-echo imaging with a flexible echo time. J Magn Reson Imaging 2008;27(4):881-890.
5. Berker Y, Franke J, Salomon A, Palmowski M, Donker HC, Temur Y, Mottaghy FM, Kuhl C, Izquierdo-Garcia D, Fayad ZA, Kiessling F, Schulz V. MRI-based attenuation correction for hybrid PET/MRI systems: a 4-class tissue segmentation technique using a combined ultrashort-echo-time/Dixon MRI sequence. J Nucl Med 2012;53(5):796-804.
6. Jang H, Ma Y, Carl M, Jerban S, Chang EY, Du J. Ultrashort echo time Cones double echo steady state (UTE-Cones-DESS) for rapid morphological imaging of short T2 tissues. Magn Reson Med 2021;86(2):881-892.
7. Kronthaler S, Boehm C, Feuerriegel G, Bornert P, Katscher U, Weiss K, Makowski MR, Schwaiger BJ, Gersing AS, Karampinos DC. Assessment of vertebral fractures and edema of the thoracolumbar spine based on water-fat and susceptibility-weighted images derived from a single ultra-short echo time scan. Magn Reson Med 2021;87(4):1771-1783.
8. Feuerriegel GC, Kronthaler S, Boehm C, Renz M, Leonhardt Y, Gassert F, Foreman SC, Weiss K, Wurm M, Liebig T, Makowski MR, Schwaiger BJ, Karampinos DC, Gersing AS. Diagnostic value of water-fat-separated images and CT-like susceptibility-weighted images extracted from a single ultrashort echo time sequence for the evaluation of vertebral fractures and degenerative changes of the spine. Eur Radiol 2022.
9. Kronthaler S, Feuerriegel GC, Boehm C, Gersing AS, Schwaiger BJ, Makowski MR, Weiss K, Karampinos DC. On the robustness of single UTE-Dixon for simultaneous short T2*, water and fat imaging across skeletal anatomies. Proc ISMRM 2022. p 536.
10. Krishnamoorthy G, Willmering MM, Woods JC, Pipe JG. High-quality Lung imaging with FLORET UTE and Fibonacci interleaved trajectory ordering. Proc ISMRM 2022. p 3497.
11. Pipe JG, Zwart NR, Aboussouan EA, Robison RK, Devaraj A, Johnson KO. A new design and rationale for 3D orthogonally oversampled k-space trajectories. Magn Reson Med. 2011;66(5):1303-1311.
Figures