0119
Spherical Echo-Planar Time-resolved Imaging (sEPTI) for 3D highly-accelerated, distortion-free, time-resolved whole-brain T2* mapping1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Ming Hsieh Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 4Department of Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
Keywords: Data Acquisition, Data Acquisition, Echo-planar imaging, time-resolved imaging
EPTI is a rapid time-resolved quantitative imaging method. In this work, we developed a spherical EPTI sampling trajectory (sEPTI) to improve its speed. To achieve fast imaging, sEPTI traverses a tight 3D spherical k-space using full ramp-sampling and variable echo-spacing, which also desirably increases its spatiotemporal incoherency. sEPTI was demonstrated in vivo to provide improved imaging performance over conventional block 3D-EPTI that requires 1.4x longer scan; achieving high-quality 1mm isotropic whole-brain proton-density and T2* maps in 48s. As part of this work, a novel and effective reconstruction approach to mitigate high-spatial-order ghosts in echo-planar acquisitions was also developed.Introduction
Echo planar time-resolved imaging (EPTI) is a rapid quantitative imaging technique1-5. Recently, circular-EPTI (cEPTI)6 was proposed for 2D imaging, which traverses a tight circular k-space coverage to provide 30-40% scan time reduction when compared with regular 2D-EPTI. In this work, we developed spherical-EPTI (sEPTI) to accelerate the scan of 3D-EPTI by covering a tight spherical k-space with varying echo spacing at different k-space locations. The proposed sEPTI achieved high-quality time-resolved T2* imaging for T2* and PD mapping across the whole-brain at 1-mm isotropic resolution in 48 seconds, and whole-brain 0.75-mm isotropic resolution in 3 minutes. As part of this work, a new reconstruction approach was developed to account for high-spatial-order even-odd ghosts, which was demonstrated to improve the reconstruction of not only EPTI but also EPI.Methods
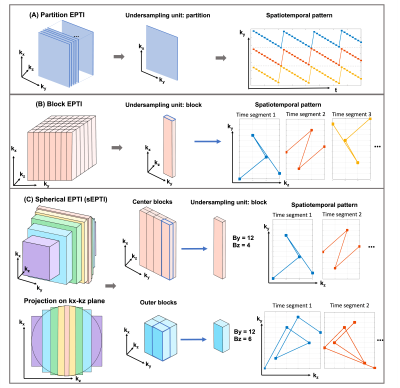
Sampling design: Different spatiotemporal sampling trajectories for gradient-echo 3D-EPTI were evaluated: i) partition-EPTI which is a direct extension of 2D-EPTI with undersampling along ky and full-sampling along kz, with 5 EPTI-shot covers one kz partition (Fig1A), ii) block-EPTI which divides the ky-kz plane into blocks, where each EPTI-shot samples ky and kz within one block using varying spatiotemporal sampling across the readout segments (Fig1B), and iii) spherical EPTI (sEPTI, Fig1C), which also acquires k-space in blocks, but with reduced number of shots achieved through: a) cutting the ky-kz corners and b) reducing kx extend at outer ky-kz positions to follow a spherical envelop which reduces echo-spacing and enable increased ky-kz block size. In this work, at 1mm resolution, the reduced number of shots in sEPTI allow for 1.4x faster than block-EPTI, with more gain expected at higher resolutions where less time is spent on ramp-sampling at low gradient amplitudes.Image reconstruction: Low-rank subspace EPTI reconstruction2,7 was implemented as
$$\widehat{\mathbf{U}}=\arg\min_{\mathbf{U}}\|\mathbf{{\Omega}FSBP}\mathbf{U}\boldsymbol{\Phi}-\mathbf{d}\|_{2}^{2}+R(\mathbf{U}),$$
Here, $$$\mathbf{U}$$$ is the spatial-coefficients to be solved, $$$\mathbf{d}$$$ is the acquired data, $$$\mathbf{\Omega}$$$ is the undersampling mask, $$$\mathbf{F}$$$ is Fourier-transform, $$$\mathbf{S}$$$ is coil-sensitivity, $$$R(\cdot)$$$ is a sparsity regularizer, $$$\boldsymbol{\Phi}$$$ is temporal subspace pre-determined from a dictionary representing a range of physical T2* decays, and $$$\mathbf{B}$$$ is the B0 induced phase. New in this work, we incorporate the eddy-current induced high-order phase ($$$\mathbf{P}$$$) between the even and odd echo lines into the forward model to mitigate image artifacts from such system imperfection. To estimate this phase ($$$\mathbf{P}$$$), a low-resolution fully sampled calibration data was acquired, which was also used to estimate coil-sensitivities and initial B0 map.
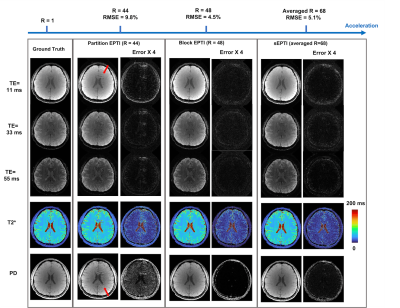
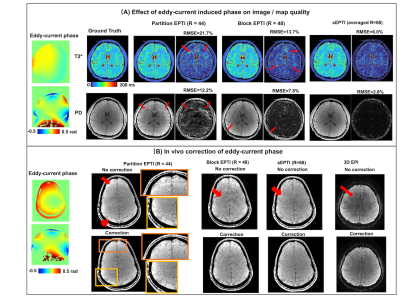
Simulations: Simulations were performed using a set of PD, T2*, sensitivity, B0, and P maps from a fully-sampled 3D EPTI data. Evaluations were performed on i) partition-EPTI with an undersampling rate R = 44, ii) block-EPTI with R = 48 (By = 12 and Bz = 4), and iiii) sEPTI with R = 48 at center k-space and R >70 for the outmost k-space. The reconstructions were performed once without and once with high-order eddy-current phase correction to evaluate its effect (DC and linear terms removed via standard ghost-correction).
In vivo experiments: Seven volunteers were studied on a 3T system (Premier, GE Healthcare) with a 48-channel head-coil. Images were acquired in sagittal with: FOV=216x216x192mm3, spatial-resolution=1.0x1.0x1.0mm3, TEs=5-60ms, TR=75ms, echo-spacing=1.1ms, flip-angle=90°. The acquisition times for partition-EPTI, block-EPTI, and sEPTI were 82s, 65s, and 48s, respectively. To demonstrate the potential of sEPTI for higher resolution, one volunteer was scanned at 0.75-mm isotropic resolution. The acquisition time was 1.5 minutes, and two averages were acquired in 3 minutes.
Results
Figure 2A shows simulation reconstructions, where partition-EPTI contains substantial aliasing-artifacts along y from high undersampling in that direction, while block-EPTI achieved markedly improved reconstructions, and sEPTI was able to produce similar image quality in 70% of the acquisition time.Figure 3A illustrates the effect of high-order eddy current phase. In simulations with no correction, partition-EPTI shows substantial artifacts which is reduced in block-EPTI reconstruction. The use of sEPTI further reduces these artifacts significantly, pointing to its robustness to eddy-current phase, likely as a result of its increased spatiotemporal incoherency due to its varying echo-spacing. Figure 3B shows results from in vivo scans where a similar trend in performance is observed. Moreover, with eddy current correction, the reconstruction performance improves for all cases. In this example, 3D-EPI was also acquired and reconstructed to show the generalizability in the benefit of the proposed eddy-current correction in also removing shading/aliasing artifacts in conventional echo-planar acquisitions.
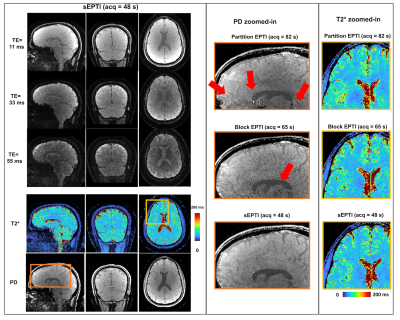
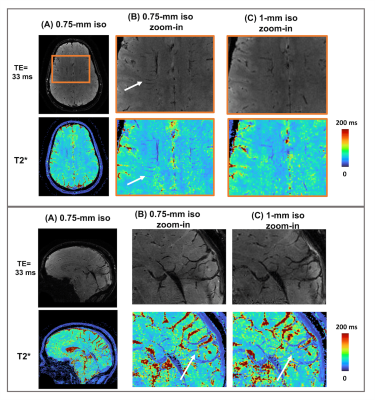
An in vivo example at 1-mm isotropic resolution is shown in Figure 4 with T2* weighted images at different TEs as well as PD and T2* maps in axial, coronal, and sagittal views. sEPTI provides better images and maps when compared to partition-EPTI and block-EPTI that requires longer scan times. Figure 5 shows sEPTI results at 0.75mm isotropic resolution, where finer delineation of brain structures is achieved.
Discussions
sEPTI showed great abilities for ultrafast quantitative brain imaging. It is robust to eddy current and potentially B0 variations due its increased spatiotemporal incoherency from varying echo-spacing at different k-space locations.Conclusion
A novel sEPTI technique was developed for ultrafast time-resolved brain imaging. It is robust to eddy-current, and achieved robust 3D whole-brain T2* and PD mapping at 1-mm isotropic resolution in 48 seconds and 0.75-mm isotropic resolution in 3 minutes.Acknowledgements
No acknowledgement found.References
1. Wang, Fuyixue, Zijing Dong, Timothy G. Reese, Berkin Bilgic, Mary Katherine Manhard, Jingyuan Chen, Jonathan R. Polimeni, Lawrence L. Wald, and Kawin Setsompop. "Echo planar time‐resolved imaging (EPTI)." Magnetic resonance in medicine 81, no. 6 (2019): 3599-3615.
2. Dong, Zijing, Fuyixue Wang, Timothy G. Reese, Berkin Bilgic, and Kawin Setsompop. "Echo planar time‐resolved imaging with subspace reconstruction and optimized spatiotemporal encoding." Magnetic resonance in medicine 84, no. 5 (2020): 2442-2455.
3. Wang, F., Z. Dong, T. G. Reese, L. L. Wald, and K. Setsompop. "3D‐EPTI for ultra‐fast multi‐contrast and quantitative imaging." Proc Intl Soc Mag Reson Med. Montreal(2019): 944.
4. Dong, Zijing, Fuyixue Wang, Lawrence Wald, and Kawin Setsompop. "SNR-efficient distortion-free diffusion relaxometry imaging using ACcelerated Echo-train shifted EPTI (ACE-EPTI)." bioRxiv (2021).
5. Dong, Zijing, Fuyixue Wang, Kwok-Shing Chan, Timothy G. Reese, Berkin Bilgic, José P. Marques, and Kawin Setsompop. "Variable flip angle echo planar time-resolved imaging (vFA-EPTI) for fast high-resolution gradient echo myelin water imaging." NeuroImage 232 (2021): 117897.
6. Wang, N, Liao C, Srinivasan, S, Cao, X, Haldar J, Setsompop K. “Circular Echo-Planar Time-resolved Imaging (cEPTI) for rapid time-resolved and quantitative imaging.” In the Proceedings of the Annual Meeting of ISMRM, London, England, UK, 2022. p 0761.
7. Liang Z-P. Spatiotemporal imagingwith partially separable functions. IEEE; 2007:988-991.
Figures