0112
High-fidelity PSF reconstruction for fast and silent MRI using nonlinear spatially encoding gradient fields switched at 20kHz1Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Spinoza Center for Neuroimaging, Amsterdam, Netherlands
Synopsis
Keywords: Gradients, Gradients
Acoustic noise in MRI scans can be reduced by utilising gradient switching frequencies at 20kHz. High slew rates required to achieve such high-frequency switching can lead to peripheral nerve stimulation (PNS), adding difficulty for application to whole-body gradients. Instead, nonlinear encoding gradients have been shown to limit PNS while achieving desired slew rates. In this work, we show the feasibility of using a nonlinear silent gradient for spatial encoding by employing a PSF-based reconstruction and investigate image fidelity on a 4-fold accelerated in-vivo scan. This method could potentially be utilised for a whole-body gradient design for silent and fast MRI.Introduction
Gradient switching causes acoustic noise, which contributes to patient discomfort in MRI. The acoustic noise arises due to gradient coil vibrations with vibration frequency proportional to the slew rate. Most attempts to reduce acoustic noise focus on gradient amplitude reduction which results in an increase in scan time1. Previously, we have demonstrated that silent spatial encoding is possible by utilising gradient switching frequencies above the human hearing limit using a gradient insert for brain MRI2. This ultrasonic encoding concept has also demonstrated significant acceleration potential similar to Wave CAIPI3. To achieve ultrasonic encoding, the head gradient insert design has a small coil diameter and length, which limits dB/dt swings allowing very high slew rates with minimal peripheral nerve stimulation (PNS)2. Extension of this method to body MRI is complex as conventional whole-body gradient coil designs will lead to large dB/dt swings and thereby PNS. However, it is possible to increase PNS thresholds by utilising shortened nonlinear gradients4. Such data can be reconstructed using a point spread function (PSF) based reconstruction which uses receive sensitivity information to resolve aliasing artefacts as a result of severe nonlinear spatial encoding5-8 . This abstract demonstrates high-fidelity image reconstruction using nonlinear spatially encoding gradients, driven ultrasonically at 20kHz. In addition, we investigate acceleration performance by retrospectively undersampling the acquired data. Our preliminary results show that this approach could be employed in a whole-body design using nonlinear gradient fields for silent and fast body MRI.Methods
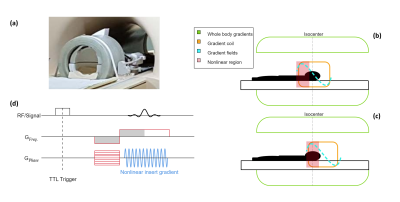
Data were acquired with a single z-axis gradient coil (Figure 1a 9). The coil was powered by an audio amplifier (Powersoft), achieving a gradient strength and slew rate of 40mT/m and 5200T/m/s, respectively. The gradient insert produces nonlinear encoding fields at the edges of the FOV (Figures 1b-c).Measurements: A head-shaped phantom containing saline water was scanned in two different positions. Firstly, the phantom was positioned in the centre (linear region) of the gradient insert that was placed in the isocentre of the whole-body gradients (Figure 1b). Secondly, the phantom was placed in the nonlinear region of the gradient insert that was placed at an offset of 110mm from the isocentre of the whole-body gradients (Figure 1c) to mimic a nonlinear gradient. Two in-vivo brain acquisitions were performed in the same positions. Fully sampled data was acquired using a 2D gradient echo sequence (Figure 1d) and the following scan parameters: TE = 11.2ms, TR = 62ms, flip angle = 22o, voxel size = 1x1x2 mm3 and an FOV of 256x400x2 mm3. Two datasets were acquired: one with the silent gradient switched on and one with the silent gradient switched off. Acceleration performance was retrospectively investigated by completing a 4-fold undersampling of the fully sampled data.
A PSF based reconstruction was used to reconstruct the data8. Here, two different reconstruction cases were tested: 1) with all gradient fields assumed to be linear, and 2) where the actual nonlinear gradient fields were used based on the field distribution of the gradient insert. SENSE reconstructions were also performed on the data acquired without the silent gradient to investigate the presence of potential artefacts caused by the silent gradient coil.
Results
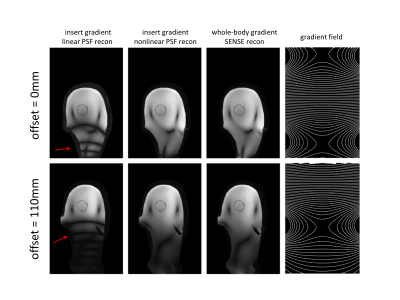
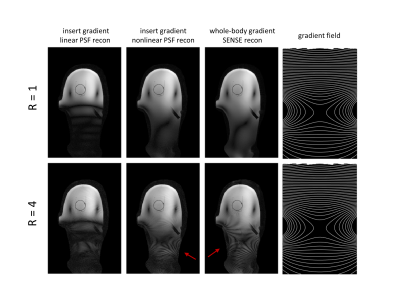
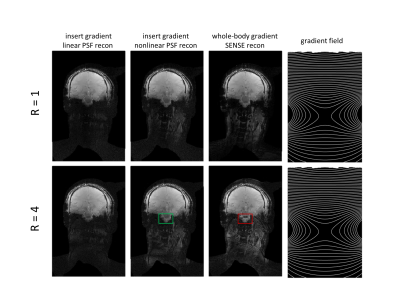
Figure 2 shows the resulting PSF and SENSE reconstructions for the phantom data for the two gradient insert offsets illustrated by figures 1b-c. Assuming nonlinear gradients during reconstruction leads to a correctly matched PSF. Data acquisition with nonlinear gradients results in multiple k-space points with the same Larmor frequency. Correct PSF matching (using the true nonlinear gradient fields) and receive sensitivity information allows these points to be separated in reconstruction. Incorrect PSF matching by assuming linear gradients resulted in banding artefacts.Figure 3 shows the comparison between reconstructions for a gradient insert offset of 110mm for fully sampled and 4-fold undersampled phantom data. Artefacts are present at a 4-fold acceleration for both PSF and SENSE reconstructions which arise from B0 field inhomogeneities in the phantom. Additionally, figures 2-3 show that high-fidelity reconstruction is possible with nonlinear spatially encoding gradients in severely nonlinear gradient regimes by utilisation of receive sensitivity information and PSF reconstruction.
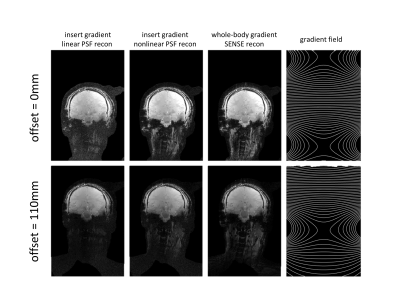
Figures 4-5 show equivalent PSF and SENSE reconstructions for in-vivo data. High-fidelity reconstruction is possible for the entire brain with nonlinear spatially encoding gradients placed in the isocentre. Lower signal is observed for the neck in the in-vivo data. As highlighted in figure 5, some artefacts were present in the SENSE reconstruction at the bottom of the brain that were not present in the PSF reconstructions as a result of the additional encoding provided by the nonlinear gradient. Higher acceleration scans are thus achievable for in-vivo PSF reconstruction using nonlinear spatially encoding gradients.
Conclusion
We have shown that high-fidelity reconstruction is feasible using silent nonlinear spatial encoding and a PSF-based reconstruction that incorporates the nonlinear fields. In addition, we demonstrated the acceleration potential for this approach. This is an initial step towards a whole-body design using nonlinear gradient fields and ultrasonic switching while avoiding PNS.Acknowledgements
This work has been financed by NWO grant number 18951.References
1. Hennel F, Girard F, Loenneker T. "Silent" MRI with soft gradient pulses. Magn Reson Med. 1999 Jul;42(1):6-10. doi: 10.1002/(sici)1522-2594(199907)42:1<6::aid-mrm2>3.0.co;2-d. PMID: 10398943.
2. Versteeg E, Klomp DWJ, Siero JCW. A silent gradient axis for soundless spatial encoding to enable fast and quiet brain imaging. Magn Reson Med. 2022 Feb;87(2):1062-1073. doi: 10.1002/mrm.29010. Epub 2021 Sep 21. PMID: 34545956; PMCID: PMC9293127.
3. Versteeg E, Klomp DWJ,Siero JCW. Accelerating Brain Imaging Using aSilent Spatial Encoding Axis. Magn Reson Med. 2022;88:1785-1793. doi: 10.1002/mrm.29350
4. Gudino N, Litten S. Advancements in Gradient System Performance for Clinical and Research MRI. J Magn Reson Imaging. 2022 doi: 10.1002/jmri.28421
5. Versteeg E, Klomp DWJ, Siero JCW. PSF-based reconstruction for removal of artifacts caused by misalignment between a silent gradient insert and the body gradients. Proc. Intl. Soc. Mag. Reson. Med. 29 2021
6. Schultz G, Ullmann P, Lehr H, Welz AM, Hennig J, Zaitsev M. Reconstruction of MRI data encoded with arbitrarily shaped, curvilinear, nonbijective magnetic fields. Magn Reson Med. 2010 Nov;64(5):1390-403. doi: 10.1002/mrm.22393. Epub 2010 Sep 16. PMID: 20848635.
7. Wang H, Tam LK, Constable RT, Galiana G. Fast rotary nonlinear spatial acquisition (FRONSAC) imaging. Magn Reson Med. 2016 Mar;75(3):1154-65. doi: 10.1002/mrm.25703. Epub 2015 May 7. PMID: 25950279; PMCID: PMC4637004.
8. Roriguez Y, Elsaid NMH, Keil B, Galiana G. 3D FRONSAC with PSF reconstruction. arXiv: https://doi.org/10.48550/arXiv.2111.05143
9. Versteeg E, van der Velden TA, van Leeuwen CC, Borgo M, Huijing ER, Hendriks AD, Hendrikse J, Klomp DWJ, Siero JCW. A plug-and-play, lightweight, single-axis gradient insert design for increasing spatiotemporal resolution in echo planar imaging-based brain imaging. NMR Biomed. 2021 Jun;34(6):e4499. doi: 10.1002/nbm.4499. Epub 2021 Feb 22. PMID: 33619838; PMCID: PMC8244051.
Figures