0110
Multi-contrast Imaging using Dual-pathway Echo-planar Imaging Sequence
Silu Han1, Sudhir Ramanna1, and Nan-kuei Chen1,2
1Department of Biomedical Engineering, The University of Arizona, Tucson, AZ, United States, 2BIO5 Institute, The University of Arizona, Tucson, AZ, United States
1Department of Biomedical Engineering, The University of Arizona, Tucson, AZ, United States, 2BIO5 Institute, The University of Arizona, Tucson, AZ, United States
Synopsis
Keywords: Pulse Sequence Design, Brain
Multi-contrast imaging, commonly acquired with spoiled gradient recalled echo (SPGR) in clinical routine examinations, has less-than-optimal scan efficiency1. Dual-pathway sequences, such as double-echo steady-state (DESS) and inverse double-echo steady-state (iDESS), have been developed to improve signal-to-noise ratio (SNR) and scan efficiency as compared with SPGR2. Here we propose to further combine DESS/iDESS sequences and echo-planar imaging (EPI) to enhance scan efficiency, with significant implications to temperature mapping and parametric mapping (e.g., T1-, T2-, T2*-mapping and B0-field mapping, quantitative susceptibility mapping (QSM)3, 4).Introduction
Multi-contrast imaging is valuable for assessing pathologically subtle changes in various tissue parameters, such as the T1-, T2-, T2*-relaxation times and the proton density (PD)1. However, clinical routine examinations for multi-contrast imaging and parametric mapping based on spoiled gradient recalled echo (SPGR) typically has limited scan efficiency. This challenge could be partially addressed by dual-pathway pulse sequences, which have proven capable of improving the temperature-to-noise ratio (TNR) and acquisition speed over standard SPGR method3, with implications in temperature mapping in MR thermometry, T1-, T2-, T2*- mapping and field mapping in quantitative imaging, and susceptibility-weighted imaging (SWI) and QSM for iron content detection associated with bleeding in the brain4.Double-echo steady-state gradient-echo (DESS-GRE) and inverse double-echo steady-state gradient-echo (iDESS-GRE) sequences, acquiring two echo-contrasts within one repetition time (TR), have the following advantages as compared with SPGR sequence2: (1) higher signal intensities due to a accumulated spin history; (2) enhanced phase contrast (e.g., with implication to SWI and QSM) due to a larger phase evolution time. However, the scan efficiency of DESS-GRE/iDESS-GRE remain less-than-optimal. To further improve scan efficiency of multi-contrast imaging and parametric mapping, here we propose innovative DESS-EPI and iDESS-EPI sequences, which integrate DESS/iDESS and EPI, to achieve optimal scan efficiency and quality.
Theory and Methods
Figure 1A shows RF and readout gradient waveforms of a DESS-GRE sequence. In the first TR, a gradient-echo (also known as “fast imaging with steady-state precession” (FISP) echo in subsequent TRs) consists of signals that experiences T2* relaxation time of TEFISP (indicated with blue double arrow in Figure 1A). In the second and all subsequent TRs, both FISP and “reverse fast imaging with steady-state precession” (PSIF) echoes exist. The PSIF signals, originating from an excitation RF pulse from the previous TR and refocusing pulse in the current TR, experience T2 relaxation time of (TR + TEPSIF), indicated with red dot double arrow in Figure 1A and additionally asymmetric spin-echo T2* duration of (TR - TEPSIF), indicated with red solid double arrow in Figure 1A. Figure 1B shows our DESS-EPI pulse sequence, in which multiple sets of FISP and PSIF echoes can be acquired in each TR with a higher scan efficiency than DESS-GRE imaging sequence. Figures 1C and 1D show iDESS-GRE and iDESS-EPI sequences, respectively, which utilize stronger pre-phasing gradients to reverse the order of FISP and PSIF echoes (in the second and all subsequent TRs). The iDESS sequences could enable longer T2* accumulation time for both FISP and PSIF echoes than DESS sequences.Using the DESS-EPI and iDESS-EPI pulse sequences shown in Figures 1B and 1D, multiple ky lines can be acquired within each TR following k-space trajectories of either Figure 2A (corresponding to larger phase-encoding blipped gradient waveforms) or Figure 2B (corresponding to smaller phase-encoding blipped gradient waveforms).
We used PulSeq framework5 to generate DESS-GRE, iDESS-GRE, DESS-EPI and iDESS-EPI pulse sequences, and acquired MRI data from healthy participants using a 3T Siemens Skyra scanner (maximum gradient: 45 mT/m and maximum slew rate: 200 T/m/s) equipped with 32-channel RF coils. Scan parameters included: FOV = 256 mm x 256 mm, matrix size = 256 x 256 x 30, axial-plane slice thickness = 3 mm and flip angle = 30$$$^{\circ}$$$. In our experiment, for DESS-EPI/iDESS-EPI, four ky lines was acquired within each TR. Different TR and echo time (TE) values were chosen. For DESS-GRE and DESS-EPI, TR/TEFISP/TEPSIF = 32.3 msec/6.1 msec/7.3 msec; for iDESS-GRE and iDESS-EPI, TR/TEFISP/TEPSIF = 31.9 msec/8.1 msec/6.9 msec. The scan duration was 5.2 min for DESS-GRE and iDESS-GRE, and 1.3 min for DESS-EPI and iDESS-EPI.
Results and Discussion
Figure 3 shows the magnitude and phase images for DESS-GRE and DESS-EPI data obtained with large phase-encoding blipped gradient waveforms. The SNR values of FISP and PSIF in DESS-GRE are 12.9 and 3.3, respectively. The SNR values increase to 19.4 (FISP) and 4.8 (PSIF) in DESS-EPI. Figure 4 shows the magnitude and phase images for iDESS-GRE and iDESS-EPI obtained with large phase-encoding blipped gradient waveforms. The SNR values of FISP and PSIF in iDESS-GRE data are 12.2 and 3.4, respectively. The SNR values increase to 19.0 (FISP) and 4.6 (PSIF) in iDESS-EPI. The scan time of DESS-EPI/iDESS-EPI is four times less than DESS-GRE/iDESS-GRE.Figure 5 shows the magnitude and phase images for DESS-EPI acquired with small phase-encoding blipped gradient waveforms after SENSE reconstruction6 and corresponding T2/T2* times.
Conclusions
We have developed an innovative dual-pathway echo-planar imaging sequences, DESS-EPI and iDESS-EPI, of high scan efficiency. Our experimental data show that high-quality MRI data can be obtained using the developed pulse sequences corresponding to various k-space scan trajectories.Acknowledgements
No acknowledgement found.References
1. Seiler A, Noth U, Hok P, et al. Multiparametric Quantitative MRI in Neurological Diseases. Front Neurol. 2021;12:640239.2. Wu ML, Chang HC, Chao TC, et al. Efficient imaging of midbrain nuclei using inverse double-echo steady-state acquisition. Med Phys. 2015;42(7):4367-4374.
3. Ciris PA, Cheng CC, Mei CS, et al. Dual-Pathway sequences for MR thermometry: When and where to use them. Magn Reson Med. 2017;77(3):1193-1200.
4. Cheng CC, Mei CS, Duryea J, et al. Dual-pathway multi-echo sequence for simultaneous frequency and T2 mapping. J Magn Reson. 2016;265:177-187.
5. Layton KJ, Kroboth S, Jia F, et al. Pulseq: A rapid and hardware-independent pulse sequence prototyping framework. Magn Reson Med. 2017;77(4):1544-1552.
6. Blaimer M, Breuer F, Mueller M, et al. SMASH, SENSE, PILS, GRAPPA: how to choose the optimal method. Top Magn Reson Imaging. 2004;15(4):223-236.
Figures
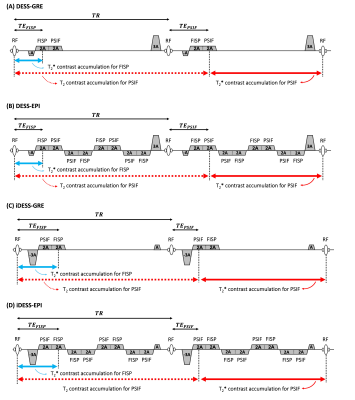
Figure 1. RF and readout gradient waveforms of DESS-GRE (A), DESS-EPI (B), iDESS-GRE (C) and iDESS-EPI (D)
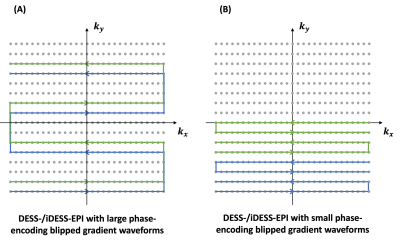
Figure 2(A) shows the DESS-/iDESS-EPI k-space acquisition trajectory with large phase-encoding blipped gradient waveforms. Figure 2(B) shows the DESS-/iDESS-EPI k-space acquisition trajectory with small phase-encoding blipped gradient waveforms.
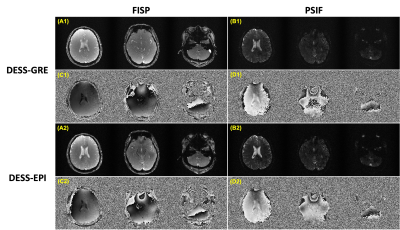
Figure 3. The magnitude and phase images of DESS-GRE and DESS-EPI (acquired with large phase-encoding blipped gradient waveforms). Column 1 shows the magnitude images of FISP (A1) and PSIF (B1) for DESS-GRE. Column 2 shows the corresponding phase images of FISP (C1) and PSIF (D1). Column 3 shows the magnitude images of FISP (A2) and PSIF (B2) for DESS-EPI and column 4 shows the corresponding phase images of FISP (C2) and PSIF (D2).
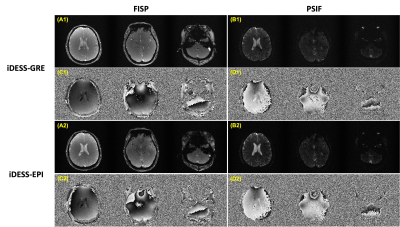
Figure 4. The magnitude and phase images of iDESS-GRE and iDESS-EPI (acquired with large phase-encoding blipped gradient waveforms) from the same subject in Figure 3. Column 1 shows the magnitude images of FISP (A1) and PSIF (B1) for iDESS-GRE. Column 2 shows the corresponding phase images of FISP (C1) and PSIF (D1). Column 3 shows the magnitude images of FISP (A2) and PSIF (B2) for iDESS-EPI and column 4 shows the corresponding phase images of FISP (C2) and PSIF (D2).
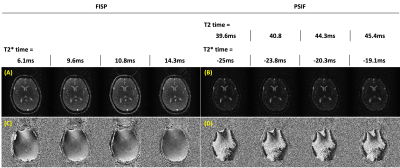
Figure 5. DESS-EPI acquired with small phase-encoding blipped gradient waveforms.
DOI: https://doi.org/10.58530/2023/0110