0109
A 3D dual-echo spiral sequence for dynamic susceptibility contrast imaging1Barrow Neurological Institute, Phoenix, AZ, United States, 2Mayo Clinic, Rochester, MN, United States, 3Philips Healthcare, Houston, TX, United States, 4The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Keywords: Pulse Sequence Design, DSC & DCE Perfusion, spiral, perfusion, quantitative imaging
Dynamic susceptibility contrast (DSC)-MRI is an important tool to assess brain tumor status for diagnosis, surgical planning, and surveillance. Dual-echo EPI based DSC-MRI enables accurate perfusion measurements using a single-dose of contrast agent, simultaneous DSC- and dynamic contrast enhanced (DCE)-MRI measures and greater parameter flexibility. However, EPI-related distortion artifacts impair its clinical utility. In this work, we proposed a 3D dual-echo spiral acquisition to mitigate EPI-related artifacts, and improve the fidelity of DCE-MRI derived parameters and arterial input function estimation. Preliminary data from volunteers and a glioma tumor patient showed reduced geometric distortion, increased temporal SNR, and accurate perfusion measurements.Introduction
Relative cerebral blood volume (rCBV) measurements from dynamic susceptibility contrast (DSC)-MRI are a critical component of brain tumor patient management 1-4.To achieve accurate DSC-MRI, a preload dose was previously recommended to reduce sensitivity to T1 leakage effects 5. While a single-dose option with low flip angle and one echo time was recently recommended 6, recent studies have shown the advantages of dual-echo sequences include elimination of T1 leakage effects, higher rCBV accuracy across a range of pulse sequence parameters, and simultaneous acquisition of dynamic contrast enhanced (DCE) MRI data 7. Recently the accuracy of dual-echo DSC-MRI for robust rCBV mapping has been validated using a digital reference object 8 and patient studies 9. However, a limitation of current dual-echo approaches is their reliance upon EPI readouts that are prone to susceptibility-induced distortion artifacts that may overlap with the location of brain tumors. In addition, the use of EPI readouts necessitates long TRs (> 500ms), which leads to insufficient T1 weighting and consequently reduces the sensitivity of this approach for robust DCE-MRI use. The present study aims to refine this dual-echo acquisition approach.
In spiral MRI, off-resonance effects exhibit themselves as blurring rather than warping in EPI, thus geometric accuracy is preserved, enabling more accurate co-registration with high-resolution anatomic scans used for surgery and radiotherapy. In this work, we developed a 3D dual-echo spiral method for accurate DSC-MRI. A multi-shot approach decouples the choice of TEs (to optimize R2* sensitivity) from the choice of resolution and coverage, and exhibits far less coherent flow and motion artifacts than multi-shot EPI. The very short TE of the first spiral echo also allows more accurate characterization of T1 changes needed for DCE-MRI. In this preliminary work, the 3D dual-echo spiral sequence was implemented and tested on volunteers to evaluate image quality and temporal SNR (tSNR), and on a patient with a glioma to verify its accuracy.
Methods
A multi-shot 3D dual-echo spiral technique (Fig. 1a) with a spiral staircase trajectory 10 was developed on a Philips Ingenia 3T scanner. To accelerate the scan, three complementary approaches were applied: i) SENSE 11 in the slice encoding direction with RSENSE = 2; ii) a variable density spiral readout designed to fully sample the center of k-space with radius r = 0.3 and undersample the outer k-space with Rspiral = 2 (Fig. 1b); iii) a sliding-window approach in the dynamic direction by sharing undersampled high-frequency k-space data among adjacent dynamic scans. The sliding-window approach is coupled with spiral undersampling in the second approach, and enabled by relatively rotating all spiral interleaves in every other dynamic acquisition (Fig. 1b). To reconstruct the images, the sliding-window reconstruction was first applied to combine high-frequency k-space data from adjacent dynamic scans, resulting in fully sampled in-plane spiral data (Fig. 1b). Then an iterative conjugate gradient algorithm was used to reconstruct the SENSE-accelerated spiral staircase data 10.To evaluate the performance of the 3D dual-echo spiral technique, 4 healthy volunteers were scanned without the administration of contrast agent. Each volunteer was scanned with 2D dual-echo EPI, 2D single-echo EPI, and the 3D dual-echo spiral sequence. Imaging parameters are listed in Table 1. Each data sets were acquired with 25 dynamics to assess the tSNR. One glioma tumor patient was scanned by collecting the 3D spiral data during preload injection with 85 dynamics in 2:24 min, followed by standard-of-care 2D single-echo EPI during the second injection (100 dynamics, 2:20 min). This study was approved by the IRB.
Dual- and single-echo time-courses were converted to ∆R2* curves for analysis. An automated approach was used to determine the arterial input function 12,13. Leakage correction was performed using the Boxerman-Schmainda-Weisskoff method 14. Subsequently, rCBV values were calculated from integration of the ∆R2* time-courses and normalized using normal-appearing white matter. Ktrans maps were fit using a reference T1 value (1.5s) to the extended Toft's model 15.
Results and Discussion
Fig. 2 shows representative volunteer images. Highlighted in the first image of dual-echo EPI (yellow arrows) are typical geometric distortion artifacts around the temporal and frontal lobes, which are absent in the spiral images. There is more signal loss at TE2, in both spiral and EPI images, due to intravoxel dephasing. Additionally, the 2D EPI images are subject to non-ideal slice profile and cross-talk effects, compared to 3D spiral images.Fig. 3 illustrates the tSNR maps (a) and mean tSNR across the whole imaging volume (b). The spiral images exhibit higher tSNR than EPI, due to multiple contributing factors such as excitation volume, readout trajectory, k-space coverage (acceleration), flip angle, RF spoiling, TE, TR, etc.
Fig. 4 demonstrates the quantitative perfusion measurements. The leakage-corrected rCBV (LC-rCBV) maps (a) indicate the single-dose dual-echo spiral data achieve accurate rCBV estimation that is comparable to standard-of-care double-dose single-echo EPI. In the calculated Ktrans map from dual-echo spiral data (b), the high-grade glioma in the parietal lobe can be clearly identified.
Conclusion
In summary, a single-dose 3D dual-echo spiral pulse sequence exhibits reduced geometric distortion artifacts and improved tSNR than conventional EPI, and accurate rCBV estimation, providing a promising reliable alternative to DSC-MRI for improved characterization of tumor status, therapy response assessment and clinical trial use.Acknowledgements
No acknowledgement found.References
1. Cha S, Lupo JM, Chen MH, et al. Differentiation of Glioblastoma Multiforme and Single Brain Metastasis by Peak Height and Percentage of Signal Intensity Recovery Derived from Dynamic Susceptibility- Weighted Contrast-Enhanced Perfusion MR Imaging. AJNR Am J Neuroradiol 2007;28:1078-1084.
2. Barajas Jr. RF, Chang JS, Segal MR, et al. Differentiation of Recurrent Glioblastoma Multiforme from Radiation Necrosis after External Beam Radiation Therapy with Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging. Radiology 2009;253:486-496.
3. Zhang J, Liu H, Tong H, et al. Clinical applications of contrast-enhanced perfusion MRI techniques in gliomas: Recent advances and current challenges. Contrast Media Mol Imaging 2017;20:7064120.
4. Mohan S, Chawla S, Wang S, et al. Assessment of early response to tumor-treating fields in newly diagnosed glioblastoma using physiologic and metabolic MRI: initial experience. CNS Oncol 2016;5:137-144.
5. Boxerman JL, Prah DE, Paulson ES, et al. The Role of preload and leakage correction in gadolinium-based cerebral blood volume estimation determined by comparison with MION as a criterion standard. AJNR Am J Neuroradiol 2012;33:1081-1087.
6. Boxerman JL, Quarles CC, Hu LS, et al. Concensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grase gliomas. Neuro Oncol 2020;22:1262-1275.
7. Vonken E, van Osch M, Bakker C, et al. Measurement of cerebral perfusion with dual-echo multi- slice quantitative dynamic susceptibility contrast MRI. J Magn Reson Imaging 1999;10:109-117.
8. Stokes AM, Semmineh NB, Nespodzany A, et al. Systematic assessment of multi-echo dynamic susceptibility contrast MRI using a digital reference object. Magn Reson Med 2020;83:109-123.
9. Stokes AM, Bergamino M, Alhilali L, et al. Evaluation of single bolus, dual-echo dynamic susceptibility contrast MRI protocols in brain tumor patients. J Cerebral Blood Flow & Metabolism. 2021;41:3378-3390.
10. Anderson III AG, Wang D, Pipe JG. Controlled aliasing for improved parallel imaging with a 3D spiral staircase trajectory. Magn Reson Med. 2020;84;866-872.
11. Pruessmann KP, Weiger M, Scheidegger MB, et al. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952-962.
12. Newton AT, Pruthi S, Stokes AM, et al. Improving perfusion measurement in DSC-MRI through the use of multi-echo information for AIF determination. AJNR Am J Neuroradiol 2016; 37: 1237–1243.
13. Carroll TJ, Rowley HA and Haughton VM. Automatic calculation of the arterial input function for cerebral perfusion imaging with MR Imaging1. Radiology 2003; 227:593–600.
14. Boxerman, J. L., Schmainda, K. M. & Weisskoff, R. M. Relative Cerebral Blood Volume Maps Corrected for Contrast Agent Extravasation Significantly Correlate with Glioma Tumor Grade, Whereas Uncorrected Maps Do Not. Am. J. Neuroradiol. 27, 859 (2006).
15. Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 1997; 7: 91–101.
Figures
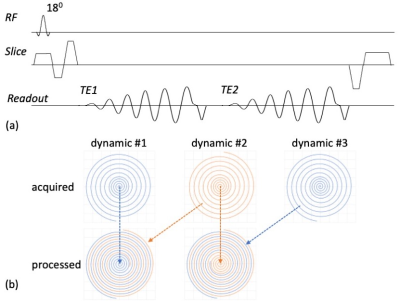
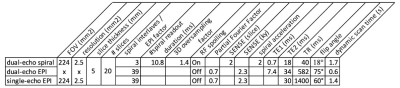
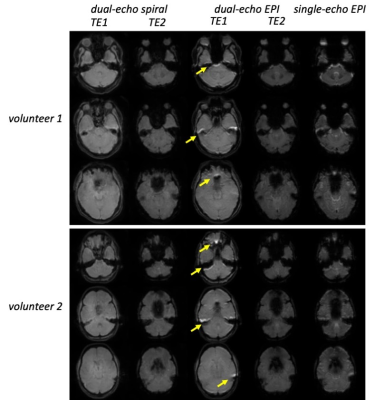
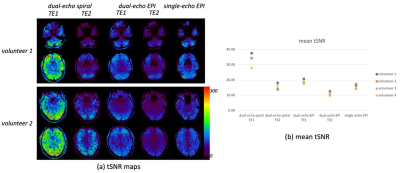
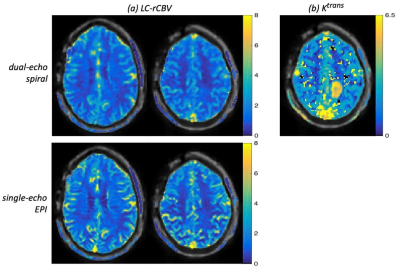
Fig. 4 Quantitative perfusion measurements from a patient: (a) leakage corrected (LC)-rCBV maps for dual-echo spiral and single-echo EPI; (b) Ktrans maps for dual-echo spiral. The rCBV values from dual-echo spiral are comparable to those from standard-of-care single-echo EPI, demonstrating its accuracy. In the Ktrans map from dual-echo spiral data, the high-grade glioma in the parietal lobe is clearly identified.