0103
Association Between Ultrashort Echo-Time T2* Parameters and Clinical Outcome in an RCT of Patellar Tendinopathy1Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3Massachusetts General Hospital/Harvard Medical School, Boston, MA, United States, 4Department of Orthopedics and Rehabilitation, University of Wisconsin-Madison, Madison, WI, United States, 5Department of Biostatistics and Medical Informatics, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States
Synopsis
Keywords: Tendon/Ligament, Quantitative Imaging, UTE
This study was performed to investigate the association between bi-component UTE-T2* parameter of the patellar tendon and clinical pain scores over time in a randomized control trial for patellar tendinopathy (PT). The fraction of the fast-relaxing macromolecular bound water (FF) of the proximal patellar tendon and VAS pain scores were assessed at 0, 16 and 52-weeks in 29 patients with PT randomized into three treatment groups. All treatment groups showed a significant improvement (p<0.05) in pain and a significant increase (p<0.05) in FF over time, with a significant inverse correlation (p<0.05) between the increase in FF and decreased pain.Introduction
Patellar tendinopathy (PT) is a common degenerative condition in active individuals characterized by pain within the proximal patellar tendon leading to a lengthy and often incomplete recovery1–3. Ultrashort echo-time (UTE) techniques can capture rapidly decaying signal within tendon and may be able to assess disease-related and treatment-related changes in PT4. Bi-component UTE T2* mapping has been used to improve the specificity of UTE T2* analysis by evaluating the individual water components of tendon, including the fast relaxing fast-relaxing macromolecular bound water and slow-relaxing bulk water5–7. This study was performed to investigate the association between single-component and bi-component UTE-T2* parameters of the patellar tendon and clinical pain scores over time in a double-blinded randomized control trial (RCT) of three interventions for PT. A secondary aim was to investigate the association of UTE T2* parameters with conventional and shear-wave ultrasound (US) parameters of the patellar tendon.Methods
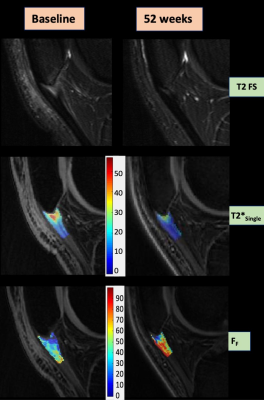
PT was diagnosed by a sports medicine specialist using standardized clinical criteria with the diagnosis confirmed with US. Patients with PT were block randomized into three treatment groups: platelet-rich plasma (PRP), dry needling (DN), and sham (SH).VAS pain scores and conventional US measures including tendon thickness, hypo-echogenicity, and hyperemia were acquired in all subjects at 0, 16 and 52-weeks. Shear-wave US of the proximal patellar tendon was performed to measure shear wave speed (SWS), a proxy for tendon elasticity. A sagittal three-dimensional (3D) gradient-echo-based multi-echo UTE-T2* mapping research sequence (3D-Cones, GE Healthcare, Waukesha, WI) was performed on the knee of all subjects at 0, 16, and 52-weeks using a 3.0T scanner (Discovery MR750, GE Healthcare, Waukesha, WI) and 8-channel phased-array extremity coil (InVivo, Orlando, FL). The sequence utilized a 3D cone k-space sampling scheme with 16 TEs between 0.003ms and 35ms. Additional imaging parameters included a 40ms repetition time, 20° flip angle, 16cm field-of-view, ±150KHz bandwidth, 256×256 matrix, 3mm slice thickness, one excitation, 10 slices through the patellar tendon, and 19-minute scan time.
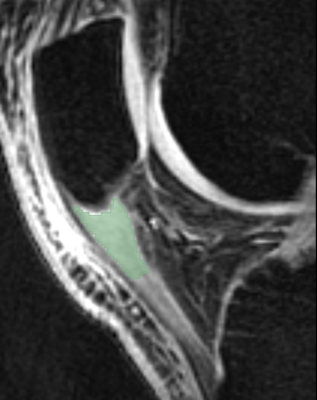
A musculoskeletal radiologist delineated regions of interest (ROIs) of the proximal 1cm of the patellar tendon using 3D Slicer on the UTE images with TE of 4.3ms. All echoes were registered to this echo using rigid registration8. Single-component and bi-component UTE-T2* signal models based on non-linear least square “fmincon” function incorporating Rician noise were used to create 3D maps of single-component T2* relaxation time (T2*Single) and bi-component T2* parameter maps including the T2* relaxation time of fast-relaxing macromolecular bound water (T2*F), T2* relaxation time of slow-relaxing bulk water (T2*S), and the fraction of fast-relaxing macromolecular bound water (FF)9. The ROIs of the proximal patellar tendon were superimposed over the parameter maps to calculate mean and standard deviation values.
Longitudinal data analysis with subject as a random effect were used to compare between group and within group differences in mean outcome measures over time. The square root of the coefficient of determination from mixed effects linear regression was used to assess correlations between outcome measures.
Results
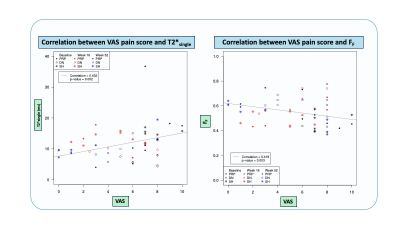
29 patients (83% male, mean age 26.1yrs ± 5.3) were included in the RCT and randomized into the following groups: PRP=9; DN=11; and SH=9. There was a significant improvement in pain in all groups from baseline to 52 weeks with the PRP group having the most pain relief (Δ-5.87 95%CI [-7.84,-3.89] p<0.001).There was a significant increase in FF from baseline to 52-weeks for all groups (Δ=0.10-0.11, 95%CI [0.00-0.20], p=0.024-0.046), but a significant decrease in T2*Single over time only in the PRP group (Δ=-8.07, 95%CI [-14.58,-1.55], p=0.014). There was no significant change in T2*F and T2*S over time.
An increase in FF was significantly correlated with decreased pain (r=-0.42, p=0.003) and decreased tendon hyperemia (r=-0.49, p<0.001), while a decrease in T2*Single was significantly correlated with decreased pain (r=0.43, p=0.002) and decreased tendon thickness (r=0.55, p<0.001). There was no significant correlation between the UTE-T2* parameters and SWS.
Discussion
Our study demonstrated the responsiveness of UTE-T2* parameters, especially the bi-component parameter FF, in a RCT in patients with PT. All treatment groups showed a significant improvement in pain and a significant increase in FF of the patellar tendon over the 52-week follow-up period, with a significant inverse correlation between the increase in FF and decreased pain over time. PT is characterized by a decrease in collagen content, increase in bulk water content, and collagen disorganization10,11, with the pathologic changes in the patellar tendon associated with a significantly (p<0.001) lower FF compared to healthy tendon (9). The increase in FF with treatment of PT in our study indicates a reversal of these tissue-level changes and likely reflects an increase in collagen content and a decrease in bulk water content of the patellar tendon during the healing response. T2*Single also demonstrated responsiveness to treatment of PT but to a lesser extent than FF. This may be due to the non-specificity of T2*Single to the individual constituents of the patellar tendon as it merely represents a composite measure of the T2 relaxation times and fractions of the different water components.Conclusion
UTE-T2* parameters, especially the bi-component parameter FF, are promising MRI biomarker to assess healing changes in the patellar tendon in PT.Acknowledgements
We would like to acknowledge the National Basketball Association-General Electric Sports Medicine Collaborative for funding this study, Bracco Diagnostics for providing research support to the department of Radiology of the University of Wisconsin-Madison and our research nurse Jan Yakey for the effort she put in recruiting, screening, enrolling, consent, blood draw, patient safety, and follow up of the patients in this study. We also want to acknowledge the grants NIBIB R21EB031185, NIAMS R01AR079442 and R01AR081344 for providing research support to Fang Liu.References
1. Fredberg U, Stengaard-Pedersen K. Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation. Scand J Med Sci Sports. 2008 Feb;18(1):3–15.
2. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005 Apr;33(4):561–7.
3. Kettunen JA, Kvist M, Alanen E, Kujala UM. Long-term prognosis for jumper’s knee in male athletes. A prospective follow-up study. Am J Sports Med. 2002 Oct;30(5):689–92.
4. Chang EY, Du J, Chung CB. UTE imaging in the musculoskeletal system. J Magn Reson Imaging JMRI. 2015 Apr;41(4):870–83.
5. Pauli C, Bae WC, Lee M, Lotz M, Bydder GM, D’Lima DL, et al. Ultrashort-echo time MR imaging of the patella with bicomponent analysis: correlation with histopathologic and polarized light microscopic findings. Radiology. 2012 Aug;264(2):484–93.
6. Du J, Diaz E, Carl M, Bae W, Chung CB, Bydder GM. Ultrashort echo time imaging with bicomponent analysis. Magn Reson Med. 2012 Mar;67(3):645–9.
7. Kijowski R, Wilson JJ, Liu F. Bicomponent ultrashort echo time T2* analysis for assessment of patients with patellar tendinopathy. J Magn Reson Imaging JMRI. 2017 Nov;46(5):1441–7.
8. Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010 Jan;29(1):196–205.
9. Liu F, Kijowski R. Assessment of different fitting methods for in-vivo bi-component T2* analysis of human patellar tendon in magnetic resonance imaging. Muscles Ligaments Tendons J. 2017 Mar;7(1):163–72.
10. Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatol Oxf Engl. 2004 Feb;43(2):131–42.
11. Xu Y, Murrell GAC. The basic science of tendinopathy. Clin Orthop. 2008 Jul;466(7):1528–38.
Figures