0100
Lower limb muscle-water T2 as a marker of disease severity and progression in amyotrophic lateral sclerosis1Queen Square Institute of Neurology, University College London, London, United Kingdom
Synopsis
Keywords: Muscle, Quantitative Imaging
Using optimised maximum-likelihood-estimation extended-phase-graph-model relaxometry in ALS patients, elevated muscle water T2 (T2m) were revealed for all calf muscles and most thigh muscles vs. controls, with evidence of sparing of particular muscles. Similar but les consistent increases were seen in the associated fat fraction (ffa, relaxometry estimated). T2m correlated significantly with both functional rating scores and myometry, most consistently at the calf level. Associated ffa changes and correlations were less significant.
Introduction
Lower limb muscle-water T2 (T2m) has been shown in amyotrophic lateral sclerosis (ALS) to be elevated vs. controls, with suggested associations between T2m and progression1. Here we also analysed multi-echo MRI data, obtained as part of a wider study from which a first report described elevated fat fraction and STIR hyperintensity particularly at calf level in ALS2. We investigated lower-limb T2m vs. healthy controls at baseline, and longitudinally, correlating T2m changes and effective fat fraction (ffa) with functional assessment and myometry.Methods
Participants, recruited with informed consent, comprised people with ALS (n=20, age 57.3 ±14.8y) and healthy controls (CTR) (n=16, age 55.4 ±13.5y). Participants were assessed using the ALS Functional Rating Scale–Revised (ALSFRS-R)3. Lower limb myometry was obtained with a microFET®2 handheld dynamometer (Hoggan Scientific; UT, USA) for isometric assessment of knee and ankle extension and flexion. Patients were examined at baseline, 6 and 12 months, controls at baseline and 12 months.MRI was performed at 3T (Siemens Skyra) with a multi-echo spin-echo sequence (TR= 3630/3500ms, 22 TEs from 10-220ms with 10ms interval, 9 x 6 mm slices, matrix 320x160, in-plane resolution 1.3125x1.3125 mm). A multi-component, slice profile-corrected EPG model [s(TE) = (1 - ffa) · sEPG(B1f, T2m, α, σN, TE) + ffa · [ 0.33 · sEPG(B1f, T2=40ms, α, σN, TE) + 0.67 · sEPG(B1f, T2=198ms, α, σN, TE)] was fitted pixel-wise to the data using maximum likelihood estimation in MATLAB, to estimate T2m and an apparent fat fraction ffa. The 2-component fat-signal model parameters were estimated a priori from 4 subcutaneous fat ROIs in 8 representative subjects. Muscles were manually segmented on single slices at thigh and calf level. Mean T2m and ffa were calculated for all individual muscle ROIs, for the anterior and posterior compartments, and the entire musculature cross section at each level.
Results
All calf muscles showed significant T2m increases (one way ANOVA, p<0.05 considered significant) in patients vs. controls. At the thigh level T2m differences were more variable: Table 1 shows thigh-level T2m and ffa group differences at baseline and 1 year. Significant differences are shown in green. For most thigh muscles T2m was significantly elevated in ALS vs. CTL, although the sartorius and gracilis appeared largely spared. Significant differences for ffa were less consistent.A tendency to negative correlation was seen between both T2m and ffa and ALSFRS-R which reached significance at the calf level (entire cross-section left and right combined) at baseline and 6 months. The correlations were stronger for T2m compared with ffa (p values: 0.002 and 0.002 vs 0.046 and 0.047, R values - 0.68 and - 0.58 vs - 0.47 and - 0.50, for baseline and 6 months respectively). Figure 1 shows the correlation of calf T2m and ALSFRS-R at baseline.
In the patients, anterior and posterior muscle compartment T2m and ffa at thigh and calf levels at baseline, 6 months and 1 year were tested for correlation with the respective myometry results. Correlations were significant for a number of these (Table 2), most consistently for T2m at the calf level, with p<0.05 for 9 out of 12 correlations (4 compartments at the 3 time points).
Myometry changes between baseline and 6 months were significant for all compartments (p < 0.039). Concomitant T2m changes were significant for all calf compartments (p < 0.036) and for left anterior thighs (p = 0.002); ffa changes were significant for all left limb compartments (p < 0.014).
Four muscle compartments showed significant correlation between longitudinal T2m (ΔT2m) change and myometry changes (ΔMyometry) (Figure 3). Analogous correlations for all other muscle compartments were not significant.
Discussion
Consistent with previous reports, in most muscles at calf and thigh level T2m was at baseline higher in patients than in controls. Notable exceptions were the sartorius and gracilis for which patient T2m were close to control values.T2m and ffa also showed significant negative correlation with ALSFRS-R scores at the calf level. This somewhat contrasts with previous results1 where significant positive correlation was observed at thigh level. Differences in these findings may be due to differences in the specific patient groups, or technical differences in the T2m estimation method4.
Several muscle compartments showed significant negative correlation between T2m and ffa, and myometry, most notably T2m in the calf and longitudinal T2m change correlated significantly with muscle strength change in several compartments, suggesting T2m reflects functionally relevant cellular changes.
Conclusion
In addition to baseline differences of T2m between patients and controls, associations between T2m and ALFRRS-R and myometry results support the validity of T2m as a disease biomarker. These data provide some evidence of a stronger association between T2m and function than was seen for ffa.This approach using routinely available CPMG pulse sequences provides T2m and ffa estimates from a single acquisition which provide complementary indices of neuromuscular pathology.
Acknowledgements
This work was supported in part by GSK and the UCLH NIHR biomedical research centreReferences
1. Paoletti, M., et al., Longitudinal Quantitative MRI Evaluation of Muscle Involvement in Amyotrophic Lateral Sclerosis. Frontiers in Neurology, 2021: p. 1998.
2. Klickovic, U., Zampedri, L., Sinclair, C.D., Wastling, S.J., Trimmel, K., Howard, R.S., Malaspina, A., Sharma, N., Sidle, K., Emira, A. and Shah, S., 2019. Skeletal muscle MRI differentiates SBMA and ALS and correlates with disease severity. Neurology, 93(9), pp.e895-e907.
3. Cedarbaum, J.M., et al., The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. Journal of the neurological sciences, 1999. 169(1-2): p. 13-21.
4. Zafeiropoulos, N. Reducing parameter estimate bias due to B1 field error ambiguity in CPMG MRI T2 relaxometry. in ISMRM. 2020. Virtual Conference.
Figures
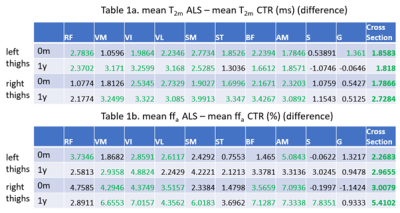
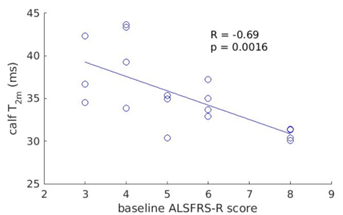
Figure 1. Correlation between the ALSFRS-R lower limb score and T2m at baseline at the calf level.
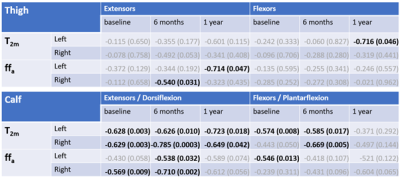
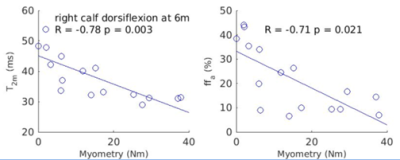
Figure 2. Example plots (for right calf flexors T2m and ffa vs. dorsiflexion torque) where both T2m and ffa correlated significantly with myometry.
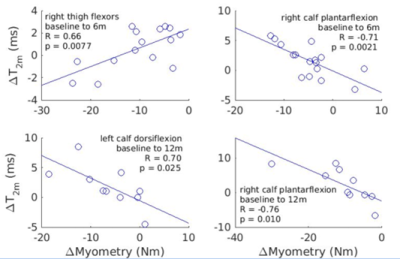
Figure 3. Plots from muscle compartments for which longitudinal T2m changes (ΔT2m) and myometry changes (ΔMyometry) showed significant correlation.