0090
Microcirculation in aging skeletal muscle assessed with flow-compensated Intravoxel Incoherent Motion (IVIM)1Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
Keywords: Muscle, Diffusion/other diffusion imaging techniques
Assessment of microcirculation is extremely important in skeletal muscle aging reasearch. While IVIM can provide extremely valuable information on perfusion, this is usually dominated by flow in larger vessel, resulting in limited sensitivity to microcirculation. In this study we propose the use of flow and non-flow-compensated diffusion encoding waveforms for IVIM of skeletal muscle. Using this approach we show diffrences in IVIM parameters between younger and older adults, and show correlation between the difference in IVIM parameters and ageIntroduction
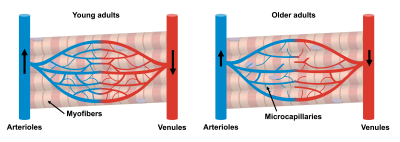
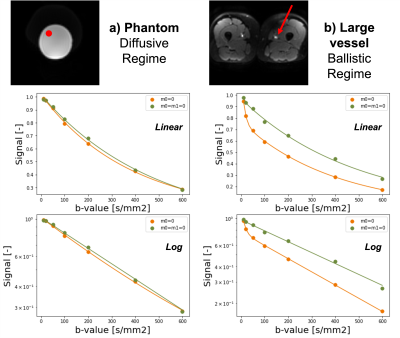
Muscle tissue is extensively affected by the aging processes. The progressive decline in muscle mass and strength in older adults poses a large economic burden(1). Microcapillaries (referred to as microcirculation) are responsible for supplying muscle cells with nutrients and removing waste products, and are suspected to play a key role in the aging processes(2). Furthermore, detection of changes in skeletal muscle microcirculation could predict subsequent (and often irreversible) atrophic changes and therefore inform physical intervention strategies, but are currently difficult to detect non-invasively (Figure 1). According to the IVIM model, perfusion (blood flowing through capillaries) in skeletal muscle can be measured by fitting a bi-exponential decay model to a diffusion multi-shell acquisition. However, when larger blood vessels are present within a voxel, they dominate the perfusion signal, therefore hiding the microcirculatory contribution. The main assumption of the IVIM model is that perfusion can be considered a pseudo-diffusion process. However, this assumption breaks when larger blood vessels (compared to the diffusion time) are present within a voxel. The use of diffusion-encoding gradients with flow compensation could eliminate the signal decay coming from longer capillary segments (Figure 2), and therefore allow the study of microcirculation parameters. The aim of this study was to implement an M0+M1 compensated diffusion encoding strategy to evaluate skeletal muscle microcirculation and apply it to an aging population.Methods
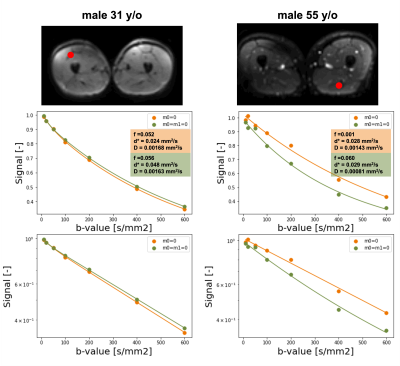
Both thighs of 10 subjects (mean age =48 y/o, 5 female ) were imaged with a 3T SIGNA Premier scanner (GE HealthCare, Waukesha, WI). We acquired two IVIM datasets: one without flow compensated (M0=0) and one with flow compensated (M0=M1=0) diffusion encoding gradients, applied along 3 orthogonal directions. Other imaging parameters were kept constant: TR/TE = 3000/65 ms, matrix: 126x126x16, voxel size= 3.6x3.6x10 mm3, b=10, 20,50,100,200,400,600 s/mm2. The TE for each diffusion encoding waveform, determined by the acquisition with b = 600 mm2/s and M0=M1=0, was minimized using a real-time gradient optimization of the CODE framework (3,4). All image processing steps were performed in python using Dipy (4). All datasets were first concatenated and denoised using a Local PCA algorithm. Registration to the non-flow-compensated scan acquired at b = 600 s/mm2 was applied to correct for subject motion. A two-step segmented approach (trr) was used for IVIM fitting (boundaries: 0<f<1, 0.005<d<1, 0<D<0.005). For each dataset, data were extracted in two ROI located in the quadriceps and hamstring muscles, and differences were calculated pixelwise between parameters obtained with M0=0 and M0=M1=0.Results
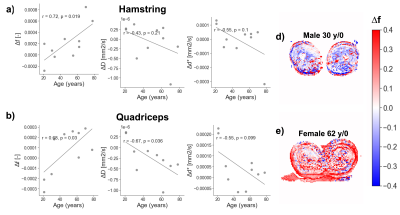
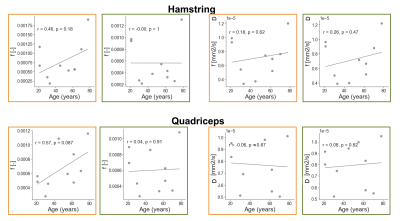
Biexponential fitting of the thigh was successful for all subjects. We observed different decay profiles when using flow-compensated and non-flow-compensated waveforms (Figure 3). The difference in perfusion between different acquisitions correlated positively with age for both hamstring and quadriceps muscles. The difference in diffusion coefficient D correlated negatively with age for the quadriceps, and had a similar pattern for the hamstring. We also observed a decreasing trend for the differences in d* as a function of age, but they did not reach significance (Figure 4). Interestingly, none of the two IVIM acquisitions alone revealed any correlation with age (Figure 5), although f (M0=0) showed a trend. This highlights the importance of multiple acquisitions to isolate different perfusion components.Discussion
In this study, we exploited the combination of flow-compensated and non-flow-compensated diffusion gradients to quantify microcirculation in old and young adults at rest. Previous studies have utilized IVIM (5) or Arterial Spin Labeling (ASL) to detect perfusion changes in the lower extremities after exercise or occlusions, therefore requiring two different acquisitions. On the other hand, our method does not require an external intervention to change perfusion and is more specific to smaller blood vessels that are in the diffusive regime.In areas with compromised microvasculature, we observed a large positive difference between the two acquisitions. However, against intuition, we did identify several areas where the signal from the flow-compensated acquisition was decaying faster than with the simple M0=0 gradients. We believe this may results in the reduced average displacement of the water molecules in the blood when flow-compensated waveforms are used. This agrees with previous research which showed time dependence in the IVIM signal in rodent brain tissue (7).
In this study the diffusion time was not kept constant between acquisitions, and our results suggest that this likely played a role in the observed signal decay and modeling. Future research will focus on studying the time dependence of the IVIM signal, to obtain more detailed information on the geometry of the underlying microstructure.
Despite these limitations, this was the first study to our knowledge to show the added value of flow-compensated diffusion gradients in IVIM of human musculature, and our findings suggest that we can disentangle (at least partially) microscopic blood flow and microvasculature. This is supported by the strong correlation between Δf and age.
In conclusion, we have shown that microvasculature in skeletal muscle can be probed non-invasively and without requiring an exercise paradigm. These findings could facilitate the stratification of subjects at risk of developing sarcopenia and provide new options for timely intervention.
Acknowledgements
No acknowledgement found.References
1. Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, Pereira SL: Economic Impact of Hospitalizations in US Adults with Sarcopenia. J Frailty Aging 2019; 8:93–99.
2. Jin K: A Microcirculatory Theory of Aging. Aging Dis 2019; 10:676–683.
3. Aliotta E, Wu HH, Ennis DB: Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion–compensated diffusion-weighted MRI. Magn Reson Med 2017; 77:717–729.
4. Loecher M, Middione MJ, Ennis DB. A gradient optimization toolbox for general purpose time‐optimal MRI gradient waveform design. Magn Reson Med 2020;84(6):3234-45.
5. Garyfallidis E, Brett M, Amirbekian B, et al.: Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 2014; 8(FEB):1–17.
6. Adelnia F, Shardell M, Bergeron CM, et al.: Diffusion weighted MRI with intravoxel incoherent motion modeling for assessment of muscle perfusion in the thigh during post-exercise hyperemia in younger and older adults. NMR Biomed 2019; 32:e4072.
7. Wu D: Evidence of the diffusion time dependence of intravoxel incoherent motion in the brain. Magn Reson Med. 2019; 82:91859201.
Figures