0079
The joint registration of multiple microscopy contrasts to MRI in the BigMac dataset1Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2University of Oxford, Oxford, United Kingdom
Synopsis
Keywords: Data Processing, Data Processing, registration, microscopy, histology, postmortem, macaque
We built a novel pipeline to register 290 high-resolution multi-contrast microscopy sections in the BigMac dataset to post-mortem MRI with sub-millimetre accuracy. The MRI-microscopy alignment is optimised over all microscopy sections jointly, to achieve high-quality co-registration whilst ensuring the slide order and separation is consistent with how the tissue was sectioned. We demonstrate the accuracy of the registration by overlaying FreeSurfer-estimated grey-white matter contours on orthogonal sections of 3D volumetric reconstructions of each microscopy modality. Our pipeline provides an integrated workflow for diffusion-microscopy studies using the BigMac dataset, which is openly available via the Oxford Digital Brain Bank.Introduction
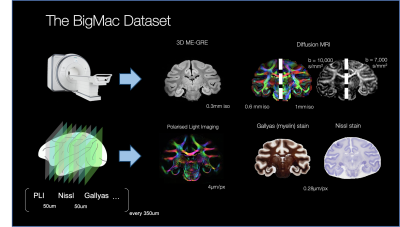
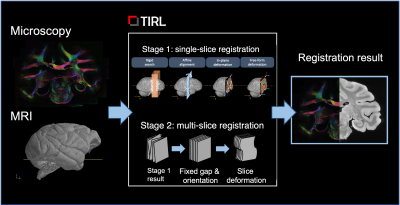
Combined MRI-microscopy datasets are increasingly in demand, as they can provide novel insight to the cellular-level constituents of MR contrasts, as well as ground truth for validating diffusion microstructural models of the brain. The BigMac dataset (Fig.1) [1-3] is a unique collection of in vivo MRI, high-quality postmortem MRI (structural, relaxometry and diffusion data) and multi-contrast microscopy in a single, whole macaque brain. The microscopy data acquired so far includes coronal sections of polarised light micrographs (PLI), and myelin- and Nissl-stained histology can shed light onto the complete cyto- and myeloarchitecture of the macaque brain. Here, we report the development of a high-performance image registration pipeline for the simultaneous alignment of multiple microscopy contrasts to post-mortem MRI in the BigMac dataset. Existing histology-MRI registration methods tend to rely on the reconstruction of a 3D stack of microscopy data by registering consecutive sections of a single modality to each other [4] or to a series of blockface photographs [5]. Our TIRL-based [6] pipeline computes direct microscopy-to-MRI transformations for each slice, which not only makes it straightforward to use with multi-modal microscopy data, but also to work with microscopy images at native resolution.Methods
For this work, we selected 290 microscopy sections (96 Nissl, 95 Gallyas, 99 PLI) from the anterior half of the BigMac brain for registration with the structural MRI (multi-gradient echo, 0.3 mm/voxel isometric). Our registration method utilises information about how the tissue was sectioned to constrain the optimisation: that Nissl, Gallyas and PLI slices were acquired in order, with a nominal separation of 50 um (the thicknesss of each section), and sections with the same microscopy contrast were repeated 350 um throughout the brain.The registration consisted of two stages (Fig.2). In Stage 1, each slice’s position and orientation in MRI space were optimised independently from other slices, given a starting estimate. The optimisation process involved a highly parallelised grid search over the 6 rigid parameters, followed up by gradient-free BOBYQA [7] optimisations at 0.6mm and 0.3mm resolutions to minimise the Modality-Independent Neighbourhood Descriptor (MIND) metric [8].In Stage 2, the pre-registered slices were recruited successively in batches of 60-120-180…, starting from the most well-aligned slice. For each batch, a robust slice orientation was determined based on the outlier-free group average, and the 6 rigid parameters were re-optimised from this new initial value at 0.15mm resolution. After the last batch, the slice orientations were replaced by the robust group average and held constant, while the positions of the slices were reoptimized one final time, using small amounts of regularisation to favour nominal slice separations. Finally, in-plane deformations were optimised using Gaussian radial basis functions with 8 even-spaced control points.Volumetric reconstructions of the three modalities were performed by interpolating the data of the registered slices in MRI space on a 0.3-mm voxel grid using barycentric linear interpolation. The grey-white matter surface was reconstructed from MRI data using FreeSurfer [9] and overlaid on the microscopy volumes for a visual assessment of the registration.Finally, we measured the average displacement of brain pixels on all slices between Stage 1 and Stage 2, to obtain a quantitative estimate of Stage-1 accuracy as a stand-alone histology-to-MRI registration method.Results
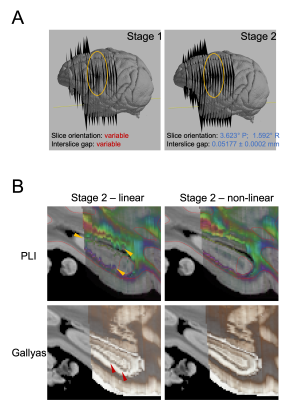
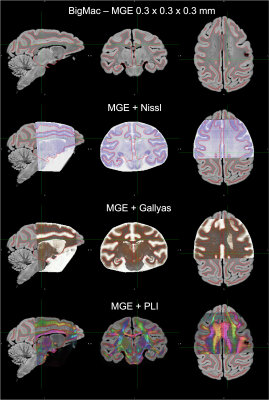
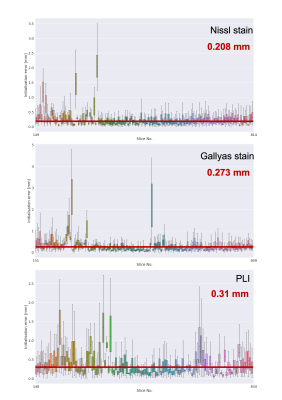
Matching anatomical contours were observed on coronal slices after the MRI data was resampled onto the pre-registered microscopy image domains (Stage-1 result). A 3D visualisation after Stage 1 revealed that slight differences in the orientation of neighbouring slices resulted in consecutive slices intersecting or crossing over one-another, which is unrealistic.The additional constraints in stage 2 effectively eliminated the crossing slices and increased the accuracy of the registration. The output of stage 2 provides an estimate of the vector normal to the microscopy plane, which was found to be slightly off-coronal (with a 3.623° posterior and a 1.592° right rotation). The constraint for parallel slides was achieved without compromising in-plane anatomical correspondence on any of the slices. The empirical slice gap was found to be 51.7um, which is in strong agreement with the nominal value of 50um, and the final position of the slices seem to strictly adhere to this regular pattern of Nissl-Gallyas-PLI sections being acquired in order. The non-linear registration step in Stage 2 successfully addressed bulk deformations of the histology slide, as shown by the improved consistency of anatomical contours of the hippocampus on sagittal sections (Fig.3).Fig.4 shows full orthogonal sections of the 3D volumetric reconstructions. The self-consistency of the reconstructed anatomy, and the alignment of the grey-white matter contours in all viewing planes confirm the high-quality registration of all slices.Finally, our quantitative analysis in Fig.5 shows that on average, Stage-1 estimates of microscopy pixel positions were offset from their Stage 2 target in MRI space by 0.208mm for Nissl-stained slices, 0.273mm for Gallyas, and 0.310mm for PLI. This indicates the accuracy of registering stand-alone sections of histology by Stage 1Conclusion
We demonstrate state-of-the-art MRI-microscopy co-registration in the BigMac dataset. These warpfields will be made open access alongside the BigMac data already available in the Oxford Digital Brain Bank. Together, these carefully co-registered MRI and microscopy data can facilitate new avenues for meaningful MRI-microscopy comparisons and multi-scale research.Acknowledgements
AFDH and KLM contributed equally to this work. SZ is supported by the Chinese Government Scholarship and the Nuffield Department of Clinical Neurosciences studentship. INH, SJ, KLM, and AFDH are supported by the Wellcome Trust (grants WT202788/Z/16/A, WT215573/Z/19/Z, and WT221933/Z/20/Z). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z).References
[1] Howard A, Jbabdi S, Krapichev AA, Sallet J, Daubney G, Mollink J, Scott C, Sibson N, Miller K. The BigMac dataset: ultra-high angular resolution diffusion imaging and multi-contrast microscopy of a whole macaque brain, 2019, Proceedings of the 27th Annual Meeting of the ISMRM
[2] Howard AF, et al. The microscopy connectome: towards 3D PLI tractography in the BigMac dataset. ISMRM 28th Annual Meeting, 2020
[3] Howard AF, et al. The BigMac dataset: an open resource combining multi-contrast MRI and microscopy in the macaque brain. bioRxiv, 2022
[4] Kyle CT, Stokes J, Bennett J, Meltzer J, Permenter MR, Vogt JA, Ekstrom A, Barnes CA. Cytoarchitectonically-driven MRI atlas of nonhuman primate hippocampus: Preservation of subfield volumes in aging. Hippocampus. 2019 May;29(5):409-421. doi: 10.1002/hipo.22809. Epub 2017 Nov 17. PMID: 29072793; PMCID: PMC5920786.
[5] Choe AS, Gao Y, Li X, Compton KB, Stepniewska I, Anderson AW. Accuracy of image registration between MRI and light microscopy in the ex vivo brain. Magn Reson Imaging. 2011 Jun;29(5):683-92. doi: 10.1016/j.mri.2011.02.022. Epub 2011 May 5. PMID: 21546191; PMCID: PMC3100355.
[6] Huszar IN, et al. Tensor Image Registration Library: Automated Deformable Registration of Stand-Alone Histology Images to Whole-Brain Post-Mortem MRI Data. bioRxiv, 2022
[7] Powell, M.J. (2009). The BOBYQA algorithm for bound constrained optimization without derivatives. Online resource: https://www.damtp.cam.ac.uk/user/na/NA_papers/NA2009_06.pdf, Accessed: 10-Nov-2021
[8] Heinrich MP, Jenkinson M, Bhushan M, Matin T, Gleeson FV, Brady SM, Schnabel JA. MIND: modality independent neighbourhood descriptor for multi-modal deformable registration. Med Image Anal. 2012 Oct;16(7):1423-35. doi: 10.1016/j.media.2012.05.008. Epub 2012 May 31. PMID: 22722056. [9] Dale, A.M., Fischl, B., Sereno, M.I., 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179-194.
Figures