0065
Non-invasive evaluation of anti-integrin αvβ6 treatment of liver fibrosis by collagen-targeted MRI1Athinoula A. Martinos Center for Biomedical Imaging, Institute for Innovation in Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Pliant Therapeutics, Inc., South San Francisco, CA, United States
Synopsis
Keywords: Liver, Liver, liver fibrogenesis
A collagen-binding molecular MR probe CM-101 was employed to non-invasively assess liver fibrosis and the anti-fibrotic effects of a novel αvβ6/αvβ1 integrin antagonist treatment in bile duct-ligated rats. Reduction of fibrosis was detected by collagen imaging and correlated with morphometric assessment of fibrosis from histology. CM-101 enhanced MR is a useful tool to monitor drug treatment response in chronic liver disease.Introduction
Liver fibrosis, as a consequence of many chronic liver diseases, is a major determinant of morbidity and mortality in liver diseases.1 There is an unmet need for antifibrotic treatments which can prevent, halt or reverse hepatic fibrosis. However, drug development efforts in anti-fibrotic treatment have been hampered by a lack of non-invasive methods to detect and stage fibrosis.2 The alpha-v beta-6 (αvβ6) and alpha-v beta-1 (αvβ1) integrins are established biomarkers and attractive therapeutic targets in hepatic fibrosis as they are potent activators of transforming growth factor (TGF)β1, enhancing extracellular matrix (chiefly collagen) deposition.3-4 Here, we used molecular MRI with CM-101, a type I collagen probe that directly measures fibrosis,5 to provide a non-invasive readout of anti-αvβ6/αvβ1 treatment in a rat model of liver fibrosis.Methods
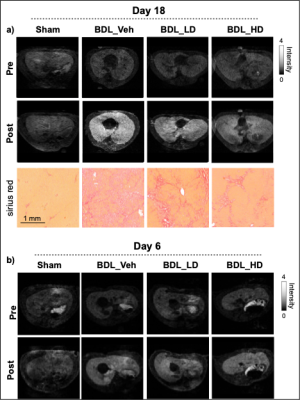
Animal study design: Liver fibrosis was induced in male Sprague Dawley rats by ligation of the common bile duct (7-9 weeks). Control animals underwent a sham procedure. The selective αvβ6/αvβ1 integrin antagonist PLN-169 was developed and provided by Pliant Therapeutics, Inc. The animals were allocated to four groups. The dosing (orally) began at day 3 post bile duct ligation (BDL) and ended at day 17. The rats were imaged with MRI probe CM-101 at day 6 and day 18. Group I included sham-operated rats (n = 6) that received vehicle twice a day (BID). Group II (BDL-veh) included rats with BDL (n = 8) receiving vehicle BID. Group III (BDL-LD) included rats with BDL (n = 9) receiving 300 mg/kg PLN-169 once per day. Group IV (BDL-HD) included rats with BDL (n = 10) receiving 500 mg/kg PLN-169 BID.In vivo imaging: Animals were anesthetized with isoflurane (1%-2%) and imaged with a 4.7T Bruker MRI scanner. Three-dimensional (3D) T1-weighted fast low-angle shot (FLASH) images (TR/TE = 20/2.3 ms; flip angle, 30°; field of view, 60×60 mm2; matrix size: 127×127; slice thickness, 0.5 mm; acquisition time, 3 minutes 10 seconds) and inversion recovery images (TR/TE = 11,000/5.6 ms; inversion time (TI) = 100, 200, 400, 500, 600, 1000, 1500 and 2000 ms; RareFactor=16; field of view, 60×60 mm2; matrix size: 140×140; slice thickness, 2 mm; acquisition time, 3 minutes 24 seconds) were acquired before and continued for 30 min after intravenous administration of CM-101 (10 µmol/kg). Following the imaging session on day 18, animals were sacrificed and liver tissue was subjected to histopathologic analysis.
Image analysis: Image visualization and quantification was performed in Horos. For T1w FLASH images, contrast to noise ratio (CNR) was calculated by subtracting the signal intensity in the muscle from that in the liver and normalizing to the standard deviation of the signal in the air outside the animal. ΔCNR was calculated by subtracting the CNRPre from CNRPost. Additionally, longitudinal relaxation time (T1) was quantified from a three-parameter nonlinear least squares fit of the dependence of liver signal intensity on TI with a custom-written MATLAB program.
Ex vivo tissue analyses: After MRI, the livers were collected, fixed, and stained with Sirius Red. Collagen proportional area (CPA) was measured using imageJ. Differences among groups were tested with one-way ANOVA test. Correlation between in vivo MR measurements and ex vivo tissue quantification was assessed by Spearman's rank correlation coefficient.
Results
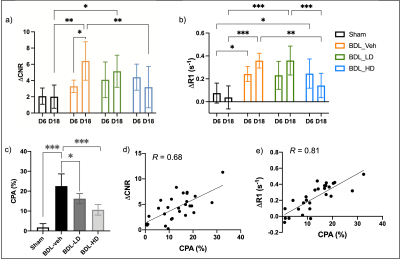
Bile duct ligation results in severe fibrosis after 18 days of surgery without treatment as assessed by CPA. Treatment with αvβ6/αvβ1 integrin antagonist PLN-169 markedly attenuated fibrosis. 3D T1w FLASH images acquired before and after CM-101 and corresponding Sirius Red staining are shown in Fig. 1a. Compared to sham animals, ∆CNR and ∆R1 in the liver was significantly increased in BDL-veh group at day 18, indicating greater uptake of CM-101 and the presence of fibrosis. Low-dose treatment with PLN-169 slightly decreased ∆CNR, while a significant decrease was observed in the high-dose treatment group. Fig. 2 shows a correlation between ∆CNR or ∆R1 and CPA. At day 6, similar elevated MRI signals compared to sham animals were observed in all BDL groups with or without treatment (Fig. 1b).Discussion
MRI results at day 6 indicates that CM-101 is sensitive in detecting early stages of fibrosis. Two weeks’ αvβ6/αvβ1 integrin antagonist treatment can effectively decrease collagen deposition in liver as revealed by CM-101 enhanced MRI at day 18. CM-101 is sensitive to monitoring the treatment responses in dose-dependent anti-fibrotic experiments and the MRI signal has a high correlation with the direct measurement of collagen/fibrosis from digital pathology (CPA). Collagen MRI can provide a noninvasive method to effectively monitor the presence of liver fibrosis and treatment response with an αvβ6/αvβ1 integrin antagonist.Conclusion
Molecular MRI of collagen can non-invasively assess liver fibrosis in a rat model of bile duct ligation, and can noninvasively report on effective anti-fibrotic treatment using an αvβ6/αvβ1 integrin antagonist. Our study raises new possibilities to non-invasively evaluate, preclinically and clinically, treatment responses in liver fibrosis and to support drug development and evaluation in this field.Acknowledgements
This work was supported by NIDDK (DK121789) and Pliant Therapeutics.References
1. Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol 2021, 18 (3), 151-166.
2. Patel, K.; Sebastiani, G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep 2020, 2 (2), 100067.
3. Popov, Y.; Patsenker, E; Stickel, F.; Zaks, J.; Bhaskar, K. R.; Niedobitek, G.; Kolb, A.; Friess, H.; Schuppan, D. Integrin αvβ6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J. hepatol. 2008, 48 (3), 453-464.
4. Reed, N. I.; Jo, H.; Chen, C.; Tsujino, K.; Arnold, T. D.; DeGrado, W. F.; Sheppard, D. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015, 7 (288), 288ra79.
5. Farrar, C. T.; Gale, E. M.; Kennan, R.; Ramsay, I.; Masia, R.; Arora, G.; Looby, K.; Wei, L.; Kalpathy-Cramer, J.; Bunzel, M. M.; Zhang, C. CM-101: type I collagen–targeted MR imaging probe for detection of liver fibrosis. Radiology. 2018, 287 (2), 581-589.
Figures