0059
Free-breathing Simultaneous T1, T2, and T2* Mapping of the Whole-Liver with Multi-Inversion Spin and Gradient Echo in Under a Minute1Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 2Radiology, University of Cincinnati College of Medicine, Cincinnati, OH, United States
Synopsis
Keywords: Liver, Quantitative Imaging
Quantitative parametric mapping is an increasingly important tool for non-invasive assessment of liver disease. Conventional parametric mapping techniques require multiple breath-held acquisitions providing limited coverage. A free-breathing multi-inversion spin and gradient echo (MI-SAGE) technique is demonstrated to simultaneously estimate T1, T2, and T2* over the whole-liver in a single scan of less than 1 minute. In 11 research participants, hepatic T1, T2, and T2* estimates obtained using the free-breathing MI-SAGE technique were comparable to those obtained using modified Lock Locker, multiple gradient and spin echo, and multiple gradient echo sequences respectively and exhibited good to excellent (ICC>0.77) repeatability and reproducibility.INTRODUCTION
Quantitative MRI is an important tool for detection, diagnosis, and evaluation of a wide range of liver pathologies1–3. However, parametric mapping of the liver can be challenging due to long acquisition times and respiratory motion. Typically, hepatic parametric maps are estimated by performing different sequence acquisitions across multiple breath-holds. This can limit the ability to co-register different parametric maps. Further, prolonged breath holds can be challenging for patients with comorbidities, and sedated patients and young children. Requirement of a breath-hold also constrains the amount of data that can feasibly be acquired. By implementing a respiratory triggered simultaneous multi-parametric acquisition, time constraints can be relaxed, and spatial resolution and coverage can be improved. Many approaches are being developed to accelerate the acquisition of parametric maps in the liver and achieve simultaneous multi-parametric hepatic maps, including MR fingerprinting (MRF)4 and MR multitasking5. The multi-inversion6–8 spin and gradient echo9,10 (MI-SAGE) method simultaneously estimates T1, T2, and T2* and has previously been applied in the brain11. Preliminary results using a breath-hold scan in the liver12 have been presented. To achieve free-breathing whole-liver T1, T2, and T2* maps in under a minute, we implemented a respiratory-triggered MI-SAGE technique, optimized to simultaneously estimate hepatic T1, T2, and T2* in 20 slices across the liver over 10 respiration cycles.METHODS
The recently developed MI-SAGE sequence11 acquires multiple contrasts differing in inversion times and echo times using single-shot echo planar imaging. Previously, the MI-SAGE technique was adapted for liver imaging in a single breath hold exam12, where simultaneously obtained hepatic T1, T2, and T2* maps were estimated for 6 slices. Here, respiratory bellows were used to detect the onset of expiration to trigger data acquisition of MI-SAGE with a specific slice order, as illustrated in Figure 1. This was followed by a pause until the subsequent expiratory trigger point, where the slice order for the MI-SAGE acquisition was shuffled6–8, continuing until the desired number of repetitions was acquired.Participants with and without known liver disease were prospectively recruited under an IRB- approved, HIPAA-compliant protocol. All imaging was performed on a 1.5T scanner (Ingenia, Philips Healthcare) using a 28-element coil. In free-breathing MI-SAGE , a total of 10 repetitions across 10 respiratory cycles were performed with shuffled interleaved slice ordering. The free-breathing MI-SAGE acquisition was repeated once for repeatability analysis, and again after repositioning the participant for reproducibility analysis. A dictionary of signal evolutions with a range of T1, T2, and T2* values were computed using the participants’ respiratory signal logs and acquisition time intervals. Dictionary matching was performed offline to each voxel over the 50 [10TIx5TE] source images to generate estimated T1, T2, and T2* maps. Scanner generated fat suppressed hepatic T1, T2, T2* and proton density fat fraction (PDFF) maps were obtained at one matching mid-liver slice with the same FOV and nominally identical acquired spatial resolution using commercially available sequences. The imaging parameters for all sequences are shown in Figure 1.
In all participants, a colocalized region of interest (ROI) was manually drawn in the right hepatic lobe at approximately matching anatomic location on all parametric maps avoiding any visible vessels and excluding the liver capsule. Mean and standard deviation of the values in the ROIs were calculated for each mapping method. Intra-class correlation (ICC) and Bland Altman difference analysis were used to assess consistency and agreement.
RESULTS
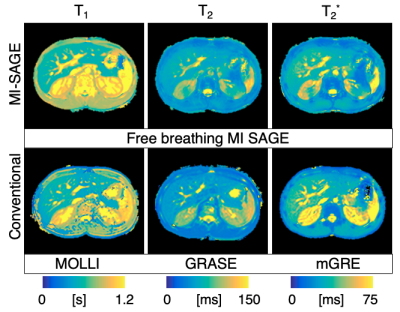
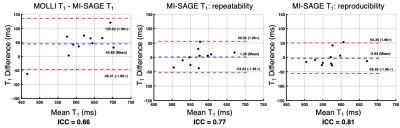
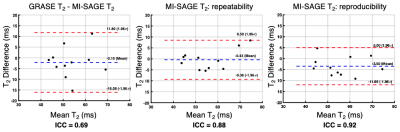
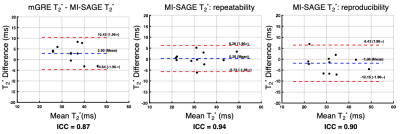
The 11 study participants ranged from 9-25 years old (4 males, 6 controls, 5 disease); liver PDFF ranged from 3-29% (median=4%). T1, T2, and T2* maps obtained with free-breathing MI-SAGE covered the entire liver volume over 20 slices in a single scan under a minute, with mean T1 = 573 (range 504-683) ms, mean T2 = 56 (range 41-80) ms, and mean T2* = 31 (range 15-54) ms. Figure 2 shows a mid-liver slice for one participant with T1, T2, and T2* maps obtained using free-breathing MI-SAGE and commercially available breath-hold mapping techniques. Figure 3 shows Bland-Altman difference plots comparing T1 MI-SAGE to A) the modified Look-Locker (MOLLI), B) repeated MI-SAGE showing repeatability, and C) a repeated MI-SAGE after repositioning, showing reproducibility. Figure 4 and Figure 5 show similar Bland-Altman analysis for T2 with multiple gradient and spin echo (GRASE), and T2*, with multiple gradient echo (mGRE) respectively. When comparing to conventional methods, MI-SAGE derived T1, T2, and T2* estimates had biases of 44.60, -2.15, and 2.90 ms, respectively. Repeated MI-SAGE measurements show little bias, with good to excellent ICC values (0.77-0.94).DISCUSSION
Hepatic T1, T2, and T2* values were estimated simultaneously across the entire liver in less than a minute using the proposed free-breathing MI-SAGE approach. Values were comparable to those obtained using conventional MOLLI, GRASE, and mGRE sequences individually acquired as separate breath-holds. Quantitative maps can be estimated over a range of T1, T2, T2*, and PDFF values with good to excellent repeatability and reproducibility (ICC>0.77), as seen in this study cohort. Hepatic parametric mapping using respiratory-triggered MI-SAGE allows for long T1 recovery time between slice acquisitions and significantly shorter quantitative liver MRI exams, with better volumetric coverage and comfortable free-breathing scan.Acknowledgements
No acknowledgement found.References
1. Hernando D, Levin YS, Sirlin CB, Reeder SB. Quantification of liver iron with MRI: State of the art and remaining challenges. J Magn Reson Imaging. 2014;40(5):1003-1021. doi:10.1002/jmri.24584.
2. Banerjee R, Pavlides M, Tunnicliffe EM, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60(1):69-77. doi:10.1016/j.jhep.2013.09.002.
3. Cassinotto C, Feldis M, Vergniol J, et al. MR relaxometry in chronic liver diseases: Comparison of T1 mapping, T2 mapping, and diffusion-weighted imaging for assessing cirrhosis diagnosis and severity. Eur J Radiol. 2015;84(8):1459-1465. doi:10.1016/j.ejrad.2015.05.019.
4. Jaubert O, Arrieta C, Cruz G, et al. Multi-parametric liver tissue characterization using MR fingerprinting: Simultaneous T1, T2, T2*, and fat fraction mapping. Magn Reson Med. 2020;84(5):2625-2635. doi:10.1002/mrm.28311.
5. Wang N, Cao T, Han F, et al. Free‐breathing multitasking multi‐echo MRI for whole‐liver water‐specific T 1 , proton density fat fraction, and quantification. Magn Reson Med. 2022;87(1):120-137. doi:10.1002/mrm.28970.
6. Ordidge RJ, Gibbs P, Chapman B, Stehling MK, Mansfield P. High-speed multislice T 1 mapping using inversion-recovery echo-planar imaging. Magn Reson Med. 1990;16(2):238-245. doi:10.1002/mrm.1910160205.
7. Clare S, Jezzard P. RapidT1 mapping using multislice echo planar imaging. Magn Reson Med. 2001;45(4):630-634. doi:10.1002/mrm.1085.
8. Renvall V, Witzel T, Wald LL, Polimeni JR. Automatic cortical surface reconstruction of high-resolution T1echo planar imaging data. Neuroimage. 2016;134:338-354. doi:10.1016/j.neuroimage.2016.04.004.
9. Schmiedeskamp H, Straka M, Newbould RD, et al. Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med. 2012;68(1):30-40. doi:10.1002/mrm.23195.
10. Eichner C, Jafari-Khouzani K, Cauley S, et al. Slice accelerated gradient-echo spin-echo dynamic susceptibility contrast imaging with blipped CAIPI for increased slice coverage. Magn Reson Med. 2014;72(3):770-778. doi:10.1002/mrm.24960.
11. Manhard MK, Stockmann J, Liao C, et al. A multi‐inversion multi‐echo spin and gradient echo echo planar imaging sequence with low image distortion for rapid quantitative parameter mapping and synthetic image contrasts. Magn Reson Med. 2021;(August 2020):mrm.28761. doi:10.1002/mrm.28761.
12. Manhard MK, Pednekar AS, Wang H, Tkach JA, Dillman JR. Simultaneous T1 , T2 , and T2* Mapping of the Liver in a Single Breath Hold Using Multi Inversion Spin and Gradient Echo. Proc Intl Soc Mag Reson Med 30. 2022;2287.
Figures