0055
Motion-Resolved Self-Gated Free-Breathing 3D Liver PDFF and R2* Mapping using Phase-Preserving Beamforming and Non-Rigid Motion Compensation1Radiological Sciences, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, United States, 2Bioengineering, University of California Los Angeles, Los Angeles, CA, United States, 3Pediatrics, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, United States
Synopsis
Keywords: Liver, Fat
3D self-navigated multi-echo stack-of-radial Dixon sequence has been used to quantify fat and R2* with free-breathing acquisitions. To compensate motion, motion-resolved compressed sensing (CS) uses self-navigation for data binning, and applies sparsity constraint along the dimension of motion states. However, this approach does not explicitly model non-rigid motion in the liver. In addition to artifacts caused by respiratory motion, hardware imperfection such as gradient nonlinearity can lead to artifacts and affect the image quality. In this work, use a phase-preserving beamforming-based coil sensitivity estimation method and non-rigid motion compensation in a CS model to improve free-breathing PDFF and R2* quantification.
Introduction
3D self-navigated multi-echo stack-of-radial Dixon sequence has been used to quantify fat and R2* with free-breathing acquisitions in fatty liver patients1-3. To compensate motion, motion-resolved compressed sensing (CS) MRI is a popular framework4 that uses self-navigation for data binning, and applies sparsity constraint along the dimension of motion states. However, this approach does not explicitly model non-rigid motion in the liver. Recently, there are studies investigating CS models that incorporate non-rigid motion information to improve sharpness in cardiac imaging5 and pulmonary MRI6. This approach has not yet been investigated in free-breathing liver fat/R2* quantification.In addition to artifacts caused by respiratory motion, previous works also suggested that hardware imperfection (e.g., gradient non-linearity) can lead to streaking artifacts7,8. These streaking artifacts usually come from the arms (closer to the peripheral field of view) and the problem becomes worse when coupled with arm movements. A phase-preserving beamforming-based method have been proposed to suppress these streaking artifacts while maintaining phase fidelity.
In this work, we developed a motion-resolved CS reconstruction framework that combines the use of the beamforming-based coil sensitivity estimation method and non-rigid motion compensation for free-breathing 3D stack-of-radial MRI PDFF and R2* quantification in children. To shorten the free-breathing acquisition time, radial undersampling was also investigated.
Methods
A complete reconstruction pipeline is shown in Figure 1.Beamforming-based method for reduced streaking artifacts: 3D images with 2-fold larger field of view were reconstructed with the acquired k-space data (i.e., 2-fold readout oversampling data was used). A deep learning network (UNet) was trained with prior datasets, and used to automatically segment the body, the right arm and the left arm. Beamforming-based radial streaking reduction relies on placing “interference patches” that contain the streaking artifacts caused by hardware imperfection and/or arm movements. We used an automatic patch selection approach that chooses two patches centered at the largest signal in the two arms. Then a 3D phase-preserving beamforming-based coil sensitivity maps were calculated8 and used in CS reconstruction.
Motion-resolved CS and deformation vector field (DVF) estimation: Radial streaking artifacts from undersampling or motion will affect DVF estimation for non-rigid motion compensation. Therefore, we first reconstructed 3D multi-echo images in different motion states using motion-resolved CS reconstruction: $$\hat{x}=\underset{x}{argmin}\left\|FSx-y\right\|^{2}_{2}+\lambda_1\left\|TV^{motion}x\right\|_1+\lambda_2\sum_{echo,slice,state}{\left\|Wx_{echo,slice,state}\right\|}_1$$, where F is non-uniform fast Fourier Transform, S is beamforming-based coil sensitivity maps, x is reconstructed images, y is self-gated k-space data, TVmotion represents total variation operator along motion states, and W represents wavelet transform. After reconstruction, DVFs between target motion state (e.g., end-expiration state) and all the other states were estimated using Demons algorithms9.
CS with non-rigid motion compensation: We constructed forward and inverse motion warping operators Mt using the estimated DVFs that can transform multi-echo 3D magnitude images between the target state and motion state t. We incorporated the warping operators to form the CS problem6 $$\hat{x}=\underset{x}{argmin}\sum_{t}^{}\left\|FSM_{t}x-y_t\right\|_{2}^{2}+\lambda_1\sum_{echo}{TGV(x_{echo})}$$, where TGV represent total generalized variation. Different from motion-resolved CS, the reconstruction results contain only images from the target state.
Fat and R2* quantification: After image reconstruction, the multi-echo images were fitted to a with 7-peak fat model and a R2* term to generate PDFF and R2* maps10.
Sequences: In a HIPAA-compliant and IRB-approved study, we acquired data from 3 children (3 males, age range:10-17 years) at 3T (MAGNETOM Skyra or Prisma, Siemens Healthineers, Erlangen, Germany) using a free-breathing multi-echo gradient-echo 3D stack-of-radial sequence. Key sequence parameters included TE=[1.23, 2.46, 3.69, 4.92, 6.15, 7.38]ms, TR=8.85ms, flip angle=5°, and scan time (min:sec) 1:38-2:07 (before acceleration).
Experiments: We compared our reconstruction results using 3D stack-of-radial data with 1.5-fold radial undersampling, using Nyquist criteria. Self-gating was applied using a 40% data acceptance window. We compared: (1) Self-gated + Non-Uniform Fast Fourier Transform (NUFFT), (2) Self-gated + motion-resolved CS, and (3) Self-gated + CS with non-rigid motion compensation. For each method, we also compare reconstructions with conventional adaptive coil combination11 to beamforming-based results.
Results
Figure 2 shows the motion-resolved image and PDFF results reconstructed with and without beamforming-based method in one subject. Figures 3 and 4 compare different reconstruction results in two subjects (one with fatty liver). Residual streaking artifacts can be observed in images and quantitative maps reconstructed without the use of beamforming-based coil sensitivity maps. Incorporating non-rigid motion compensation can further suppress the artifacts compared to motion-resolved CS (Figure 3 zoomed-in patches). Figure 5 shows a coronal reformat of the same subject in Figure 3. CS methods both improve the image quality and reduce the artifacts in the quantitative maps.Discussion
We investigated the use of non-rigid motion compensation in the CS model to improve free-breathing fat and R2* quantification. Although all CS results reduce the overall radial streaking artifacts, the specific streaking artifact pattern from the arms were not fully suppressed if beamforming-based coil sensitivity maps were not used. We also show <1.5min rapid acquisition for accurate free-breathing PDFF and R2* quantification using 1.5-fold data undersampling (compared to Nyquist criteria). There are limitations in this work. Currently, there is lack of reference for fully-sampled motion-resolved images in free-breathing scans. Image quality evaluation by the radiologists will be investigated.Conclusion
We used a compressed sensing model with phase-preserving beamforming coil sensitivity map estimation and non-rigid motion compensation for improved self-navigated free-breathing PDFF and R2* quantification.Acknowledgements
We acknowledge grant support from the National Institutes of Health (R01DK124417 and U01EB031894), and research support from Siemens Medical Solutions USA, Inc. We thank the research coordinators and the MRI technologists at UCLA.References
[1] Armstrong T, Dregely I, Stemmer A, Han F, Natsuaki Y, Sung K, Wu HH. Free‐breathing liver fat quantification using a multiecho 3 D stack‐of‐radial technique. Magnetic resonance in medicine 2018;79(1):370-382.
[2] Zhong X, Armstrong T, Nickel MD, Kannengiesser SA, Pan L, Dale BM, Deshpande V, Kiefer B, Wu HH. Effect of respiratory motion on free‐breathing 3D stack‐of‐radial liver relaxometry and improved quantification accuracy using self‐gating. Magnetic resonance in medicine 2020;83(6):1964-1978.
[3] Armstrong T, Zhong X, Shih S-F, Felker E, Lu DS, Dale BM, Wu HH. Free-breathing 3D stack-of-radial MRI quantification of liver fat and R2* in adults with fatty liver disease. Magnetic Resonance Imaging 2022;85:141-152.
[4] Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD‐GRASP: golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing. Magnetic resonance in medicine, 2016;75(2):775-788.
[5] Tolouee A, Alirezaie J, Babyn P. Nonrigid motion compensation in compressed sensing reconstruction of cardiac cine MRI. Magnetic resonance imaging, 2018;46:114-120.
[6] Zhu X, Chan M, Lustig M, Johnson KM, Larson PEZ. Iterative motion‐compensation reconstruction ultra‐short TE (iMoCo UTE) for high‐resolution free‐breathing pulmonary MRI. Magnetic resonance in medicine 2020;83(4):1208-1221.
[7] Mandava S, Keerthivasan MB, Martin DR, Altbach MI, Bilgin A. Radial streak artifact reduction using phased array beamforming. Magnetic resonance in medicine 2019;81(6):3915-3923.
[8] Shih S-F, Wu HH. A Beamforming-Based Coil Combination Method to Reduce Streaking Artifacts and Preserve Phase Fidelity in Radial MRI. 30th Annual Meeting of International Society of Magnetic Resonance in Medicine. London, UK; 2022.
[9] Thirion, J-P. Image matching as a diffusion process: an analogy with Maxwell's demons. Medical image analysis 1998: 243-260.
[10] Hernando D, Kellman P, Haldar JP, Liang, ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magnetic Resonance in Medicine, 2010;63(1):79-90.
[11] Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 2000;43(5):682-690.
Figures
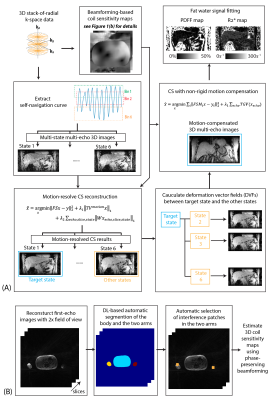
Figure 1. (a) Reconstruction pipeline for self-gated free-breathing PDFF and R2* quantification with motion-resolved CS and non-rigid motion compensation. (b) Pipeline for beamforming-based coil sensitivity map estimation with deep learning-based automatic interference patch selection.
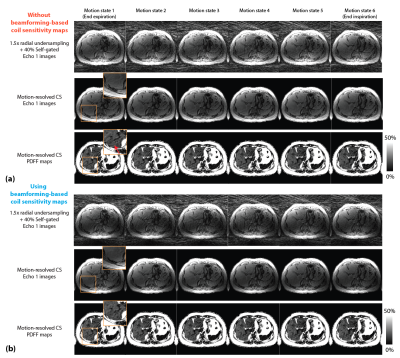
Figure 2. Representative self-gated images, motion-resolved CS reconstructed images and the corresponding proton-density fat fraction (PDFF) maps with (a) conventional adaptive coil combination, and (b) phase-preserving beamforming-based method. Residual streakings (coming from the arm outside the field of view) can be observed in CS reconstructed images and PDFF maps that did not use beamforming-based coil sensitivity maps (red arrow).
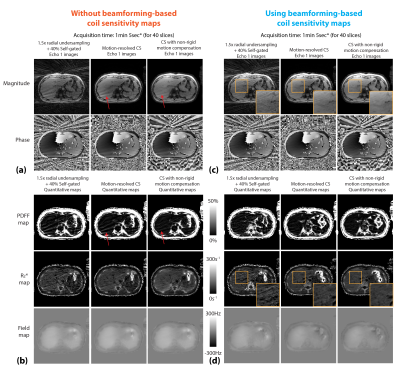
Figure 3. (a,b) Images and quantitative maps from different reconstruction methods using conventional adaptive coil combination. CS models cannot fully suppress the specific streaking patterns from the arms (red arrows). CS with non-rigid motion compensation has less streaking compared to motion-resolved CS. (c,d) Reconstruction results using phase-preserving beamforming-based coil sensitivity maps. Orange zoomed-in patches show that CS non-rigid motion compensation can better suppress the artifacts. (*pre-scan time for trajectory calibration not included.)
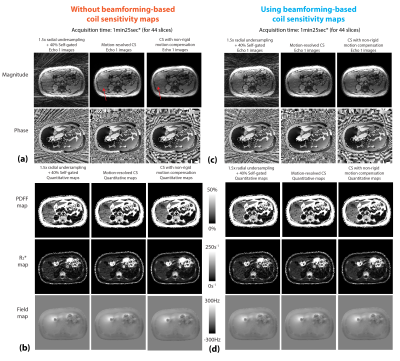
Figure 4. (a,b) Images and quantitative maps from different reconstruction methods using adaptive coil combination. CS models cannot fully suppress the specific streaking patterns from the arms (red arrows). CS with non-rigid motion compensation has less streaking compared to motion-resolved CS. (c,d) Reconstruction results using phase-preserving beamforming-based method. The image and maps show less streaking artifacts compared to results in (a) and (b). (*pre-scan time for trajectory calibration not included.)
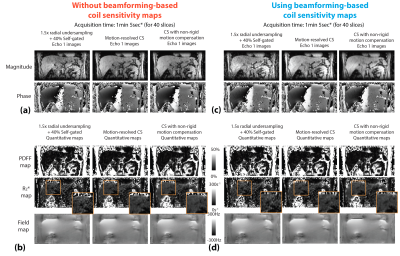
Figure 5. Comparison of images and quantitative maps in the coronal reformats from the same subject in Figure 3. CS with non-rigid motion compensation can provide results with reduced artifacts (orange zoomed-in patches). (*pre-scan time for trajectory calibration not included.)