0051
Development of safe operating procedures and early experience of scanning Newborn Infants at 7T1London Collaborative Ultra high field System (LoCUS), King's College London, London, United Kingdom, 2Guys and St Thomas’ NHS Foundation Trust, London, United Kingdom, 3Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 4MRC Centre for Neurodevelopmental Disorders, King's College London, London, United Kingdom, 5Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 6MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 7School of Biomedical Engineering and Imaging Sciences, Biomedical Engineering Department, King's College London, London, United Kingdom, 8Great Ormond Street Hospital for Children, London, United Kingdom, 9Biomedical Image Technologies, ETSI Telecomunicación, Universidad Politécnica de Madrid, Madrid, Spain, 10Department for Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar der Technischen Universität München, Munich, Germany
Synopsis
Keywords: Neuro, High-Field MRI, 7T; MRI; Newborn Infant
We describe operational processes developed for, and our first experiences in, imaging of newborn infants at 7T. Based on an initial safety study, a new SAR model was adopted, and a protocol developed for safe switching of mode prior and post imaging. Monitoring equipment was tested and cleared for use. Sequences for high-resolution and high-contrast brain imaging were optimized within stricter SAR limits. Image quality and SNR were compared at 7T and 3T, with improved anatomical and pathological features seen at 7T. Our study indicates scanning of newborn infants imaging is possible within the safety considerations needed at 7T.Background
7T MRI is increasingly being used for its improved contrast and signal-to-noise-ratio (SNR) compared to lower field strength systems(1). Neonatal scanning at 7T could be beneficial to provide additional diagnostic and prognostic information about congenital brain abnormalities or injuries sustained at birth. Whilst neonatal MRI is well documented at lower field strengths(2), regulatory barriers and a lack of safety data mean there is limited experience at 7T with only one reported study thus far(3).Our aim was to develop a comprehensive approach for imaging newborn infants at 7T. We present our safety framework and early experience in establishing brain scanning of newborn infants at 7T and report on potential benefits compared to 3T for identifying anatomy and pathology.Methods
All work was carried out using a 7T MAGNETOM Terra (Siemens Healthcare, Erlangen, Germany) with 1Tx-32Rx head coil (Nova Medical, Willington, MA, USA). This system is CE/FDA approved for patients with mass 30kg(4) only. To image newborn infants a systematic process was adopted covering MR safety, physiological monitoring, and patient handling.An RF modelling study using an in-house developed neonate model concluded that specific absorption rate (SAR) is likely to increase in newborn infants when compared with adults under the same conditions(5). Local risk assessment ascertained that suitable mitigation could be achieved by modifying scanner software to estimate higher SAR per given RF power, with an increase factor of 2.8 used to remain conservative. We implemented a standard operating procedure (SOP) for switching this factor; each time the change is made, the coil name displayed on the scanner changes, and a phantom scan is performed to verify updated SAR estimates. The safety study also suggested that close monitoring of body temperature should be used, since infants can become hypothermic if not sufficiently insulated or can experience systemic heating from prolonged RF exposure. A Philips-Invivo Expression MR400 monitor(6) was tested and verified to be used for continuous monitoring, since the manufacturer’s safety certification extends only up to 3T.
Seven newborn infants (median age of 43+2wks (range 37+6 -48+0)) were scanned at 7T and underwent 3T scanning (Achieva, Philips, Best NL) for direct comparison (NHS REC approval 19/LO/1384). Each subject was positioned supine headfirst and as centrally as possible in the RF coil with the aid of foam and inflatable pads; hearing protection was provided using dental putty and cushioning(8). They were imaged during natural sleep following feeding, and vital signs (temperature, heart rate, oxygen saturation) were monitored and reviewed by neonatal clinical staff throughout.
Structural sequences included high-resolution T2-weighted TSE images (T2w) (voxel size 0.6x0.6x1.2mm3; acquisition time 2’37”) and susceptibility-weighted images (SWI) (voxel size 0.2x0.2x1.2mm3; acquisition time 2m12s). Single-slice T1 and T2 mapping, MR spectroscopy, and functional MRI were also acquired and will be reported separately.
T2w TSE data acquired using 2-4 stacks in at least two orthogonal planes and sometimes repeated depending on presence of motion artefacts. Stacks were then combined using slice-to-volume reconstruction (SVR)(9,10) including rejection of severely artefacted slices, motion correction between odd and even slice groups, and super-resolution reconstruction with isotropic resolution 0.33-0.45mm. T2w 7T images were visually reviewed by specialist neuroradiologists to assess anatomical structures, sensitivity for any pathologies and image quality, and compared with equivalent 3T images.
Results
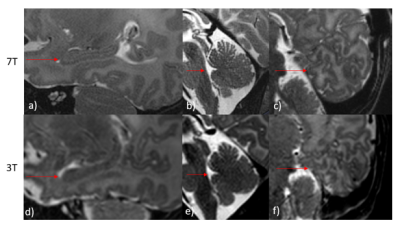
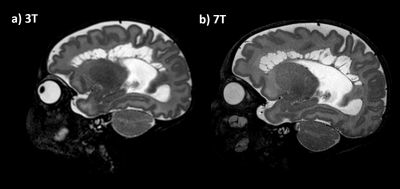
Scans were completed in all 7 infants (median time: 58.5min; scan protocol 27min48sec). Temperature and vital signs were stable throughout the scan for all participants.Across the cohort, additional detail of anatomical or pathological features were seen in the 7T scans compared to typical 3T scans. In all 7 participants, image quality of the 7T scans was observed to be equivalent or superior to 3T scans. These included: specific improvements in visualisation of the hippocampus, cerebellum vermis and cortical folding of the occipital lobe, (Figure1). 7T was also able to demonstrate pathologies and offered more information than 3T, such as improved visualization of cystic septi in periventricular leukomalacia (PVL)(Figure2).
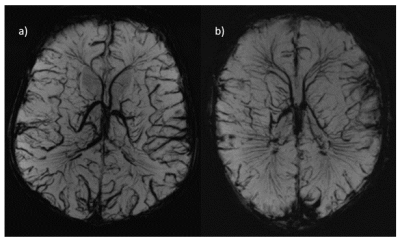
SWI at 7T allowed clear visualisation of the cerebral blood vessels as seen in Figure3. Two examples are shown, one in a participant with congenital cardiac anomalies (transposition of the great arteries) (Figure3b)), and another from a healthy control (Figure3a).
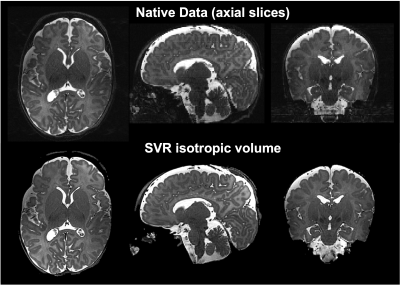
SVR of T2w images provided increased tissue contrast and SNR by combining multiple images into single 3D volumes while correcting for motion, as demonstrated in Figure4.
Discussion
Newborn infants tolerated scanning well and can be safely scanned at 7T when imaged within the more conservative safety constraints that we have enforced on the RF coil(5).In our small cohort, we found that visualisation of anatomy was better appreciated at 7T on T2w imaging in comparison to 3T, notably in the hippocampus, cerebellum vermis, and cortical folding. This may be a direct result of increased SNR. SVR was found to be helpful in both ensuring full multi-planar visualisation and in removing artefacted slices. In general, the 7T SVR results provided more details than the corresponding 3T reconstructions.
SWI at 7T also provided excellent delineation of the cerebral vasculature, however there is no direct comparison with 3T available.
Conclusion
Newborn infants can be scanned safely at 7T using appropriate safety constraints. Initial data suggests there are clear advantages for feature definition at 7T compared to 3T.Acknowledgements
This work was supported by a project grant awarded by Action Medical Research [GN2728], a Wellcome Trust Collaboration in science award [WT201526/Z/16/Z], by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health fand Social Care. TA was supported by funding from a Medical Research Council (MRC) Translation Support Award [MR/V036874/1]. ADE and TA received funding support from the MRC Centre for Neurodevelopmental Disorders, King’s College London [MR/N026063/1]. Pre-study safety testing of the Invivo Expression MR400 monitor was done in collaboration with Philips Healthcare.References
1. Oliveira ÍAF, Roos T, Dumoulin SO, Siero JCW, van der Zwaag W. Can 7T MPRAGE match MP2RAGE for gray-white matter contrast? Vol. 240, NeuroImage. 2021.
2. Dubois J, Alison M, Counsell SJ, Hertz‐Pannier L, Hüppi PS, Benders MJNL. MRI of the Neonatal Brain: A Review of Methodological Challenges and Neuroscientific Advances. J Magn Reson Imaging [Internet]. 2021 May 18;53(5):1318–43. Available from: https://onlinelibrary.wiley.com/doi/10.1002/jmri.27192
3. Annink K V., Van Der Aa NE, Dudink J, Alderliesten T, Groenendaal F, Lequin M, et al. Introduction of Ultra-High-Field MR Imaging in Infants: Preparations and Feasibility. Am J Neuroradiol. 2020;41(8):1532–7.
4. Malik SJ, Hand JW, Satnarine R, Price AN, Hajnal J V. Specific absorption rate and temperature in neonate models resulting from exposure to a 7T head coil. Vol. 86, Magnetic Resonance in Medicine. 2021. p. 1299–313.
5. Philips. Expression MR400 [Internet]. [cited 2022 Oct 8]. Available from: https://www.philips.co.uk/healthcare/product/HC866185/expression-mr400#specifications
6. Siemens. Siemens Healthineers; Magnetom Terra [Internet]. 2021 [cited 2022 Oct 8]. Available from: https://www.siemens-healthineers.com/en-uk/magnetic-resonance-imaging/7t-mri-scanner/magnetom-terra
7. Hughes EJ, Winchman T, Padormo F, Teixeira R, Wurie J, Sharma M, et al. A dedicated neonatal brain imaging system. Magn Reson Med [Internet]. 2017 Aug;78(2):794–804. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mrm.26462
8. Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, Hajnal J V., Schnabel JA. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med Image Anal [Internet]. 2012;16(8):1550–64. Available from: http://dx.doi.org/10.1016/j.media.2012.07.004
9. Deprez M, Uus A, Van Ameron J, Roberts T, Jackon L, Grigorescu I, et al. SVRTK: Slice-to-volume reconstruction toolkit [Internet]. [cited 2022 Nov 2]. Available from: https://svrtk.github.io/
Figures