0048
Comparing vascular morphology and hemodynamics in patients with Vein of Galen Malformations using intracranial 4D flow MRI1Cerebrovascular Surgery and Interventions Center, Boston Children's Hospital, Boston, MA, United States, 2Department of Neurosurgery, CSIC, Boston Children's Hospital, Boston, MA, United States
Synopsis
Keywords: Blood vessels, Blood vessels, 4D Flow, Vein of Galen Malformation, blood flow
Vein of Galen Malformation is the most common congenital cerebrovascular malformation and patients have variable mortality risk and cognitive outcomes. Tools are needed to guide treatment decisions, so we investigated the hemodynamic underpinnings of a morphological metric used for clinical prognostication. We used 4D flow MRI to quantify flow through the venous sinus that drains the malformation, and compared this to the maximum mediolateral diameter of the same sinus. A significant positive correlation between flow and diameter was observed when controlling for prior embolization; this finding motivates continued efforts to develop hemodynamic metrics of disease severity and treatment progress.Introduction
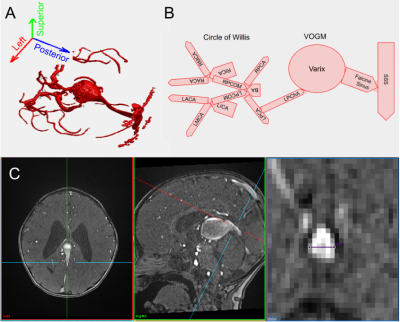
Vein of Galen Malformation (VOGM), a high flow intracranial arteriovenous shunt (Figure 1), presents in neonates or young infants as high output cardiac failure with neurological comorbidities.1,2 It is the most common congenital cerebrovascular malformation.3 Despite advances in endovascular treatments that achieve survival in selected cohorts, there is a 40% mortality rate for neonates with heart failure, and poor neurocognitive outcomes in half of survivors.4 One improvement would be the development of clear prognostic indicators for the course of the disease and eventual neurological outcomes.5 A recent effort found that the maximal mediolateral diameter of the falcine sinus at its shortest point (SS-MD) measured on fetal MRI (Figure 1B) was highly predictive of neonates who would require urgent embolizations after birth to control aggressive cardiopulmonary failure (i.e. neonatal at risk, NAR).6 Using accelerated 4D flow imaging,7 we explored the link between this anatomical measurement and intracranial blood flow dynamics to further our pathophysiological understanding of VOGM and establish a clinically useful flow-based indicator of risk and progression.Methods
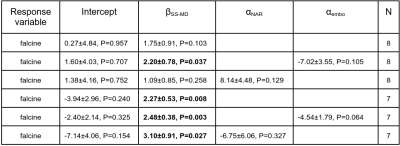
Young children with VOGM were recruited, with IRB approval, to undergo 10-15 minutes of research scanning added on to clinical scans. Research sequences consisted of phase contrast flow imaging using both 2D (2DPCMRI) and 4D approaches. 2DPCMRI was used to measure the flow in the superior sagittal sinus (SSS) upstream from the junction with the falcine sinus (Figure 1C) (repetition time (TR)=65.76ms, echo time (TE)=5.02ms, resolution=0.8x0.8x4mm3, field-of-view (FOV)=240mm, encoding velocity (VENC)=25-100cm/s through plane, 10 cardiac phases). 4D flow MRI was used to measure hemodynamics in the Circle of Willis, main venous varix and falcine sinus (TR=82.6-92.8ms, TE=3-3.2ms, resolution=1mm isotropic, FOV=220mm, VENC: low=0.6-0.8cm/s, high=1-1.6cm/s, k-t R=5, 5 cardiac phases). Manually defined vessel regions of interest were defined in Segment8 to calculate SSS flows. 4D flow data was corrected for Maxwell terms during reconstruction, and for eddy currents and velocity noise.9,10 Vessel-wise flows were then calculated in a semi-automated fashion.11 Inflow conservation error, a data quality metric,12 was calculated as (QICA-QACA-QMCA-QPCOM)/QICA for each side. Inflow was the sum of ICA and BA flow. SS-MD was measured from the TOF angiograms. We used linear models including a linear effect of SS-MD (βSS-MD) and fixed effects for embolization status (αembo) and NAR classification (αNAR), to test the relationship between these variables and falcine flow.Results
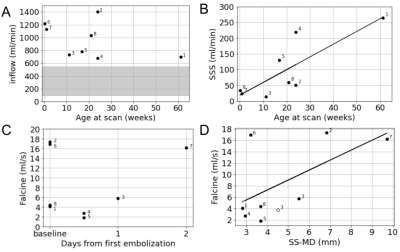
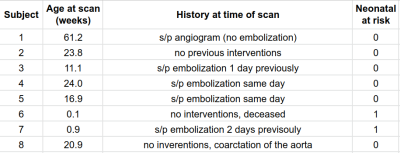
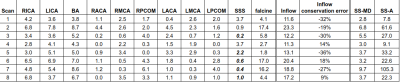
Table 1 shows the age, clinical history and NAR classification of the cohort. Table 2 presents vessel flows and SS-MD measurements which are plotted in Figure 2. Inflows are high and with no correlation to age.13,14 However, there is a significant correlation between pre-falcine SSS flow and age. Falcine flow does not correlate with age, but does correlate with SS-MD. Notably, Subject 3 was scanned again after a second embolization and was observed to have lower flow and SS-MD (Figure 2D). When considering all 8 subjects, the correlation between falcine flow and SS-MD was not statistically significant (Table 3), except when accounting for prior embolization. When Subject 6 is removed, all the models have a significant βSS-MD.Discussion
We prospectively acquired 4D flow imaging data in 8 subjects with VOGM, and found a positive correlation between falcine flow and SS-MD that was significant when controlling for prior embolizations (Table 3). This correlation was significant across all models when subject 6 was removed from the analysis. Subject 6, unusually, had a small SS-MD and high falcine flow, which may reflect his severe clinical course classified as NAR, with only moderate cardiovascular dysfunction but acute cerebral infarcts that led the clinical team to suggest palliative care only. Previous work classified NAR in ⅔ of the imaging cohort, consistent with previous reports,6 so it is also possible that our sample is not representative of the pathophysiological variety of VOGM. With a greater number of representative subjects, we could also explore the physiological mechanisms that relate SS-MD with falcine flow, since it may be a constant linear relationship only in the first approximation.Previous reports of hemodynamics in VOGM assessed with 4D flow MRI have tracked flow decrements with treatment,15,16 and Subject 3 in this study was scanned after a second embolization, which showed a proportional decrement in both falcine flow and SS-MD, matching our observed cohort trend (Figure 2D). Our observed falcine sinus flows (Figure 2C) align with a previous report.16
Our study was limited by the research scan time allowed under general anesthesia impacting obtainable image resolution. Our average inflow conservation error of 13% is in line with expected flow conservation error when <3 voxels span the vessel lumen.12
Next steps toward the development of hemodynamic metrics to guide prognosis and embolization timing include exploring the flow and pressure relationships in the draining veins, and collecting more observations from NAR patients.
Conclusion
Using 4D Flow MRI we observed a correlation between falcine sinus flow and morphology, a promising step toward developing hemodynamic metrics to guide treatment in patients with VOGM.Acknowledgements
Thanks to Maria Aristova for providing the Matlab tools and training to process the data that were collected using the 4D Flow sequence developed by Sussanne Schnell.References
1. Cordova EG, Levy P, Kheir JN, Orbach DB, Barnewolt C, Estroff JA. Vein of Galen Malformation. Neoreviews. 2020 Oct;21(10):e678–e686. PMID: 33004561
2. Raybaud CA, Strother CM, Hald JK. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology. 1989;31(2):109–128. PMID: 2664553
3. Recinos PF, Rahmathulla G, Pearl M, Recinos VR, Jallo GI, Gailloud P, Ahn ES. Vein of Galen Malformations: Epidemiology, Clinical Presentations, Management. Neurosurg Clin N Am. 2012;23(1):165–177.
4. Lecce F, Robertson F, Rennie A, Heuchan AM, Lister P, Bhate S, Bhattacharya J, Brew S, Kanagarajah L, Kuczynski A, Peters MJ, Ridout D, Schmitt A, Toolis C, Vargha-Khadem F, Ganesan V. Cross-sectional study of a United Kingdom cohort of neonatal vein of galen malformation. Ann Neurol. 2018 Oct;84(4):547–555. PMCID: PMC6221157
5. Paladini D, Deloison B, Rossi A, Chalouhi GE, Gandolfo C, Sonigo P, Buratti S, Millischer AE, Tuo G, Ville Y, Pistorio A, Cama A, Salomon LJ. Vein of Galen aneurysmal malformation (VGAM) in the fetus: retrospective analysis of perinatal prognostic indicators in a two-center series of 49 cases. Ultrasound Obstet Gynecol. 2017;50(2):192–199.
6. Arko L, Lambrych M, Montaser A, Zurakowski D, Orbach DB. Fetal and Neonatal MRI Predictors of Aggressive Early Clinical Course in Vein of Galen Malformation. AJNR Am J Neuroradiol. 2020 Jun;41(6):1105–1111. PMCID: PMC7342765
7. Schnell S, Ansari SA, Wu C, Garcia J, Murphy IG, Rahman OA, Rahsepar AA, Aristova M, Collins JD, Carr JC, Markl M. Accelerated dual-venc 4D flow MRI for neurovascular applications. J Magn Reson Imaging. 2017 Jul;46(1):102–114. PMCID: PMC5464980
8. Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment--freely available software for cardiovascular image analysis. BMC Med Imaging. 2010 Jan 11;10:1. PMCID: PMC2822815
9. Bock J, Kreher BW, Hennig J, Markl M. Optimized pre-processing of time-resolved 2D and 3D phase contrast MRI data. Proceedings of the 15th Annual meeting of ISMRM, Berlin, Germany. 2007. p. 3138.
10. Bernstein MA, Zhou XJ, Polzin JA, King KF, Ganin A, Pelc NJ, Glover GH. Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med. 1998 Feb;39(2):300–308. PMID: 9469714
11. Vali A, Aristova M, Vakil P, Abdalla R, Prabhakaran S, Markl M, Ansari SA, Schnell S. Semi-automated analysis of 4D flow MRI to assess the hemodynamic impact of intracranial atherosclerotic disease. Magn Reson Med. 2019 Aug;82(2):749–762. PMCID: PMC6510639
12. Aristova M, Vali A, Ansari SA, Shaibani A, Alden TD, Hurley MC, Jahromi BS, Potts MB, Markl M, Schnell S. Standardized Evaluation of Cerebral Arteriovenous Malformations Using Flow Distribution Network Graphs and Dual-venc 4D Flow MRI. J Magn Reson Imaging. 2019 Dec;50(6):1718–1730. PMCID: PMC6842032
13. Varela M, Groves AM, Arichi T, Hajnal JV. Mean cerebral blood flow measurements using phase contrast MRI in the first year of life. NMR Biomed. 2012 Sep;25(9):1063–1072. PMID: 22290659
14. Liu P, Qi Y, Lin Z, Guo Q, Wang X, Lu H. Assessment of cerebral blood flow in neonates and infants: A phase-contrast MRI study. Neuroimage. 2019 Jan 15;185:926–933. PMID: 29535026
15. Li Y, Ahmed R, Rivera-Rivera LA, Stadler JA 3rd, Turski P, Aagaard-Kienitz B. Serial Quantitative and Qualitative Measurements of Flow in Vein of Galen Malformations Using 4-Dimensional Flow Magnetic Resonance Imaging (Phase Contrast Vastly undersampled Isotropic PRojection). World Neurosurg. 2019 Jun;126:405–412. PMCID: PMC6924166
16. Wu C, Schoeneman SE, Kuhn R, Honarmand AR, Schnell S, Ansari SA, Carr J, Markl M, Shaibani A. Complex Alterations of Intracranial 4-Dimensional Hemodynamics in Vein of Galen Aneurysmal Malformations During Staged Endovascular Embolization. Oper Neurosurg (Hagerstown). 2016 Sep 1;12(3):239–249. PMID: 29506111
Figures