0045
Motion robust MR Fingerprinting scans for non-sedated infant imaging
Chaitra Badve1, Jessie EP Sun2, Ameya Nayate1, Michael Wien1, Douglas Martin1, Jared Durieux1, Chris Flask2, Deanne Wilson Costello3, and Dan Ma4
1Radiology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 2Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Neonatology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 4Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States
1Radiology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 2Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Neonatology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 4Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States
Synopsis
Keywords: Neonatal, MR Fingerprinting
The 5 min high resolution MRF scans coupled with low-rank iterative reconstruction successfully generated perfectly co-registered T1, T2 maps, synthetic MR contrast images, R1R2 maps, and myelin water fraction maps. Image Quality Assessment analysis with three pediatric neuroradiologists found that MRF based synthetic T1w and T2w images quality were superior in quality to MRI T1w and T2w (p<.0001) with lower image artifacts in the MRF synthetic images as compared to standard of care MRI. MRF T1w images demonstrated better myelin visualization compared to clinical T1w, and MRF T2w demonstrated improved tissue structure visualization as compared to clinical T2w images.Introduction
MR imaging of babies without sedation is challenging due to motion. Even with significant scan preparation and intensive monitoring, the imaging failure rate (with non-diagnostic images) in healthy babies can be as high as ~40%1. MRI scanning of babies with underlying health concerns can be even more challenging due to uncontrolled motion. Our institution is one of four centers participating in the Advancing Clinical Trials in Neonatal Opioid Withdrawal: Outcomes of Babies with Opioid Exposure (ACT NOW: OBOE) study2. In this cohort of neonates with opioid exposure, the scan failure rate (repeated scan rate) is staggeringly high (~56%) as these babies struggle to maintain sleep or stillness. Magnetic resonance fingerprinting (MRF) is a rapid quantitative imaging technique that shows high motion robustness and the ability to generate multiple image contrasts3. In this study, we developed an accelerated optimized MR Fingerprinting scan for non-sedated infant imaging, providing whole brain T1 and T2 maps with 0.8 mm isotropic resolution within 5 minutes of scan time. Additionally, we generated co-registered synthetic MR contrast images, R1R2 maps, and myelin water fraction maps. Finally, we performed image quality comparison analysis between MRF and MRI scans in the OBOE study subjects.Method
Recruitment: All subjects were recruited under the institutional IRB approved research protocols and were participants in the OBOE study. All infants were scanned on a Siemens Vida 3T scanner without sedation during natural sleep, or with the feed and wrap technique. All scans were monitored by a trained research coordinator and a neonatologist present in the scanner suite. Image acquisition: MRI sequences were acquired as per the OBOE study protocol. The protocol includes a 3D T2-weighted scan with 1 mm3 resolution of 2:45 minutes scan time and a 3D T1-weighted scan with 1 mm3 resolution of 3:26 minutes scan time. An MRF scan optimized by a physics-inspired algorithm4 was added at the end of all the MRI scans, with a scan time of 5 minutes and 0.8 mm3 image resolution. MRF mapping: A low-rank interactive reconstruction was performed on each MRF data, to generate whole-brain T1 and T2 maps simultaneously from a single scan. From the same data, the following additional maps and images were generated: 1) R1R2 maps, providing a unique myelin sensitivity, similar to the contrasts from the typically used T1w/T2w images; 2) synthetic MR images, such as T1w, T2w, FLAIR and DIR; 3) quantitative tissue fraction maps including gray matter, white matter and CSF fraction maps, and myelin water fraction (MWF) maps. Image quality assessment: We implemented a fully-crossed multiple reader multiple case study to assess image quality of MRF and MRI. Four image types (T1w and T2w from MRI scans, synthetic T1w and T2w from MRF) from nine neonates were randomized and rated by three board certified pediatric neuroradiologists. The image quality was assessed in three categories (image artifacts, brain structures visualization, and myelination visualization) with 16 items using a 3-point scale.Results
Multi-contrast Imaging: Figure 1 shows quantitative maps generated from an MRF scan (pt1, 10 days). Figure 2 shows synthetic MR images (A) and sub-voxel tissue fraction maps (B) generated from another subject (pt2, 15 days). Motion Tolerance, Image Quality: Figure 3A summarizes the image quality assessment rated by three pediatric neuroradiologists. For a total of 1728 observations, 67.3% of T1w and 54.6% of T2w were rated minor or no artifacts. In comparison, 71.9% of synthetic T1w and 61.1% of synthetic T2w from MRF were rated minor or no artifacts. MRF T1 and T2 image quality were superior to MRI T1w and T2w (p<.0001). Figure 3B,C demonstrates number of minor/no artifacts ratings in each category. MRF T1 consistently rated with lower image artifacts and better myelin visualization compared to clinical T1w, and MRF T2 rated consistently with lower artifacts and better tissue structure visualization categories. Figure 4 compares the image quality of the MRI T1w and T2w images and the synthetic T1w and T2w from MRF scans from two patients (pt3, 13 days, and pt4, 6 days). For pt3, the MRI T2w scan was repeated due to severe motion artifacts presented in the first scan. The repeated T2w image still showed shading artifacts due to motion. The following T1w scan was also corrupted by motion, as the periodic ghosting artifacts propagated throughout the whole brain. Pt4 is an example of when none of the repeated MRI scans provided acceptable image quality. Patient motion during the scans caused severely blurred anatomical structure and ghosting artifacts in the MRI T2w and T1w images. In comparison, the synthetic MRF T1w and T2w images from both patients were free of motion artifacts. Figure 5 compares the quantitative MRF maps from babies aged 10 days, 20 days, and 9 months. The brain structure, size, and tissue properties are substantially different. The quantitative nature of MRF maps would allow longitudinal estimation of infant brain development.Conclusion
We successfully developed a dedicated neonatal MR Fingerprinting acquisition for robust and efficient scanning in challenging non-sedated infants. The MRF-derived qualitative images offer superior image quality when compared to baby MRI scans. Additionally, the quantitative MRF maps assess baby brain development longitudinally and can potentially identify delayed or abnormal trajectories.Acknowledgements
The authors would like to acknowledge funding from Siemens Healthineers , NIH grants EB026764-01, NS109439-01, NIDA R34 DA050341-01; CWRU Planning for the Healthy Early Development Study; NICHD1PL 1HD101059-01, HEAL Initiative: Antenatal Opioid Exposure Longitudinal Study Consortium-Case Western Reserve University and The Hartwell Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.References
1. Thieba, C. et al. Factors Associated With Successful MRI Scanning in Unsedated Young Children. Front. Pediatr. 6, 146 (2018). 2. Bann, C. M. et al. Outcomes of Babies with Opioid Exposure (OBOE): protocol of a prospective longitudinal cohort study. Pediatr. Res. 1–8 (2022) doi:10.1038/s41390-022-02279-2. 3. Ma, D. et al. Magnetic resonance fingerprinting. Nature 495, 187–192 (2013). 4. Jordan, S. P. et al. Automated design of pulse sequences for magnetic resonance fingerprinting using physics-inspired optimization. Proc. Natl. Acad. Sci. U. S. A. 118, e2020516118 (2021).Figures
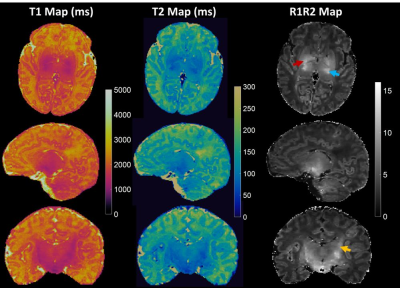
Figure 1: Whole brain T1, T2 and R1R2
maps generated from an MRF scan (pt1, 10 days). Each map defines the
appropriate myelination pattern expected in a full-term neonate. The posterior
limb of internal capsule (red arrow), lateral thalamus (blue arrow) and coronal
radiata (yellow arrow) are best highlighted on R1R2 maps.
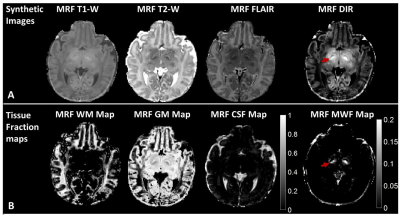
Figure 2: Synthetic MR images (A) and
sub-voxel tissue fraction maps (B) generated from a subject (pt2, 15 days). The
myelinated posterior limb of internal capsule is best defined on the DIR and
MWF images (arrowed) and not visualized on the white matter fraction maps.
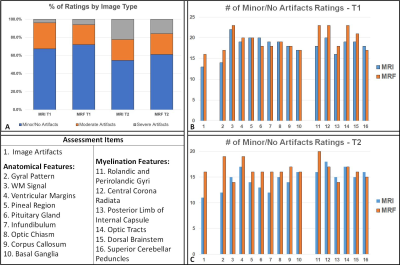
Figure 3: Image quality assessment
between T1w and T2w from MRI and synthetic T1w and T2w from MRF. (A) Summary of
the ratings (3-scale: minor/no artifacts; moderate artifacts and severe
artifacts) from three pediatric neuroradiologists for four image types. Each
image type had 9 images from 9 subjects, and each image was rated in 16
assessment items. (B,C) the number of
minor/no artifact ratings in each assessment item.
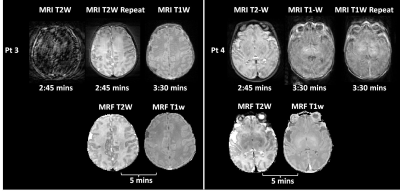
Figure 4: image quality of the MRI T1w
and T2w and the synthetic T1w and T2w from MRF from two neonate subjects. (pt3,
13 days), The first MRI T2w failed due to severe motion. The repeated T2w still
showed shading artifacts. The following T1w scan was also corrupted by periodic
ghosting artifacts due to motion. (pt4, 6 days). All the MRI scans (T2w and two
repeated T1w) did not provide acceptable image quality. The MR images were
corrupted by severely blurred structures and ghosting artifacts. As a comparison,
the synthetic MRF T1w and T2w images from both subjects were free of motion
artifacts.
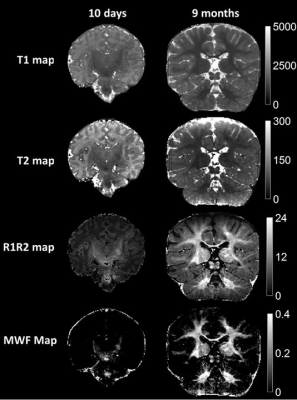
Figure 5: Comparison of the quantitative
MRF maps from babies aged 10 days and 9 months. The images demonstrate
differences in the maturation of brain structure as well as the progression of
myelination. The neonatal maps
demonstrate myelination of corona radiata, internal capsule and superior
cerebellar peduncles, while the 9 month infant maps show progression of
myelination more diffusely in the supratentorial and infratentorial white
matter.
DOI: https://doi.org/10.58530/2023/0045