0038
Brain diffusion alterations in patients with COVID-19 pathology and neurological manifestations1Bioengineering Department, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Ranica (BG), Italy, 2Department of Neuroradiology, ASST Papa Giovanni XXIII, Bergamo, Italy, 3University of Bergamo, Bergamo, Italy, 4Neurosurgical Intensive Care Unit, Department of Anesthesia and Critical Care Medicine, ASST Papa Giovanni XXIII, Bergamo, Italy, 5FROM Research Foundation, ASST Papa Giovanni XXIII, Bergamo, Italy, 6Department of Emergency and Critical Care Area, ASST Papa Giovanni XXIII, Bergamo, Italy, 7Department of Neurology, ASST Papa Giovanni XXIII, Bergamo, Italy
Synopsis
Keywords: Infectious disease, COVID-19
This study aimed at quantifying brain diffusion alterations on DWI scans in 215 COVID-19 patients with neurological manifestations as compared with 36 normal controls. The Apparent Diffusion Coefficient (ADC) was quantified in brain tissues and regions, using an in-house MRI processing procedure. In COVID-19 patients, a widespread significant increase in ADC was found in white matter. ADC values were significantly correlated with MRI time from disease onset. ADC alteration was highest in hospitalized patients. Patients with neurological disorders showed significantly higher ADC than those with olfactory loss only. DWI shows potential as a non-invasive marker of neuroinflammation in COVID-19.Introduction
Neurological manifestations have been increasingly recognized in Coronavirus disease 2019 (COVID-19).1,2 A wide range of neuroradiological findings has been reported,3–5 and the key role of brain MRI for the assessment of cerebral alterations in COVID-19 patients is being supported by increasing evidence6–8. Diffusion weighted imaging (DWI), allowing to investigate diffusion of the water molecules in the tissue, shows potential to study brain microstructure, inflammation, and edema. Previous DWI studies in COVID-19 patients reported alterations in brain diffusivity,6,9–12 as assessed by DWI morphologic evaluation. Few diffusion tensor imaging (DTI) studies confirmed significant alterations in mean diffusivity in COVID-19 survivors.13–15 This study aimed at assessing and quantifying brain diffusion alterations on DWI scans from 215 patients with COVID-19 pathology and neurological manifestations.Methods
Confirmed COVID-19 patients admitted to the ASST Papa Giovanni XXIII hospital in Bergamo, Italy, and who underwent brain MRI were eligible for inclusion. Patients with neurological disorders prior to COVID-19 and/or brain parenchymal lesions were excluded. Patients who underwent brain MRI for reasons other than COVID-19 complications with no remarkable MRI findings were considered for the control group. Brain MRI scans were acquired at the ASST Papa Giovanni XXIII hospital in Bergamo, Italy, on a General Electric 3 Tesla MRI scanner. DWI and pre-contrast T1-weighted scans were processed by an in-house semi-automatic procedure, including coregistration, grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) segmentation, computation of binary masks, registration, and DWI signal interpretation by voxel-wise monoexponential fitting. The Apparent Diffusion Coefficient (ADC) maps were restricted to GM, WM, and CSF by pertinent binary masks and to individual brain regions by available brain atlases14,16–18.Results
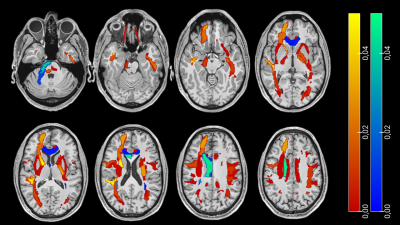
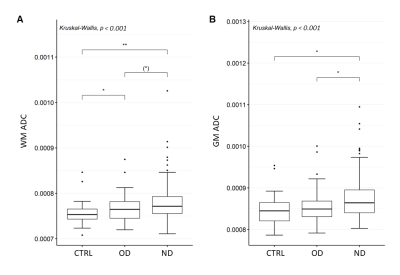
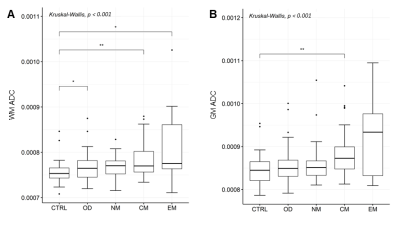
215 COVID-19 patients with neurological manifestations (52 [42 – 60] years, 58% females) and 36 controls (52 [42 – 65] years, 50% females) were included in the study. 91/215 COVID-19 patients were hospitalized, and 26/91 needed intensive care. 131 COVID-19 patients underwent brain MRI due to neurological disorders, including cognitive and memory disorders (n=68), cerebrovascular disorders (n=6), psychiatric disorders (n=9), neuromuscular disorders (n=37), encephalitis and meningitis (n=8), neuropathies (n=2) and other neurological symptoms (n=58), while the remaining 84 complained about olfactory loss only. COVID-19 patients showed significantly higher ADC values in the GM and in the WM, and significantly lower ADC values in the CSF (p < 0.05). The ADC increase involved most of the WM (Figure 1) and some GM regions, including the precentral gyrus, the rolandic operculum, the lingual gyrus, the precuneus, pallidum, and thalamus (p < 0.001). In both COVID-19 and control patients age was positively correlated with ADC values in the whole brain and each individual tissue (p < 0.001). In the COVID-19 patient group, ADC values were significantly correlated with MRI time from disease onset, in the whole brain (p < 0.001), and in individual brain tissues (p < 0.05). Hospitalized patients showed significantly higher ADC alterations than the non-hospitalized ones, in whole brain, individual tissues, and most of WM and GM regions. No significant differences in ADC values were found based on the need for intensive care. COVID-19 patients with neurological disorders showed significantly higher ADC alteration than those with hyposmia only (Figure 2). The COVID-19 patients with cognitive disorders (n = 61) showed significantly higher ADC values than the controls, in both WM and GM (p < 0.001), while COVID-19 patients with encephalitis or meningitis (n = 7) in the WM only (p = 0.035). No statistically significant increase in ADC was found in patients with neuromuscular disorders (n = 30) (Figure 3).Discussion
The reported widespread increase in WM diffusivity is in line with a recent paper15 finding diffuse axonal damage in COVID-19 patients, associated with the systemic immune-inflammation index, and suggesting a massive microglia activation, prompting neuroinflammation and causing cerebral damage. Our findings are also in line with studies showing the association between the diffusion signal and the inflammatory component in several brain diseases19,20. Consistently with this interpretation, we observed higher diffusivity in hospitalized patients and in patients with neurological disorders, that are likely denoted by higher neuroinflammation than patients with hyposmia only. Beyond WM, we found a significant ADC increase in GM, also in agreement with previous DWI and DTI studies10,11,13. The negative association observed between brain ADC and MRI time from disease onset suggests the transient nature of neuroinflammation, likely not associated with infiltration of adaptive immune cells, blood-brain barrier breakdown or cell death21. The major strengths of this study are the high number of COVID-19 patients with neurological manifestations and brain DWI included in the study, and the quantitative approach adopted for diffusion assessment. Study limitations include the small number of normal controls, the variable MRI time from disease onset, and the lack of quantitative clinical variables assessing the severity of cerebral damage.Conclusion
Current findings suggest DWI potential as a non-invasive marker of neuroinflammation in COVID-19. Future longitudinal studies are needed to investigate changes in diffusivity over time and provide definitive evidence of the transient nature of the COVID-19-related neuroinflammation.Acknowledgements
The study was supported in part by a grant from Brembo SpA (Curno, Bergamo, Italy), under the initiative "Progetto TrexUno".References
1. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767-783.
2. Sarubbo F, El Haji K, Vidal-Balle A, Bargay Lleonart J. Neurological consequences of COVID-19 and brain related pathogenic mechanisms: A new challenge for neuroscience. Brain Behav Immun Health. 2022;19:100399.
3. Choi Y, Lee MK. Neuroimaging findings of brain MRI and CT in patients with COVID-19: A systematic review and meta-analysis. Eur J Radiol. 2020;133:109393.
4. Moonis G, Filippi CG, Kirsch CFE, et al. The Spectrum of Neuroimaging Findings on CT and MRI in Adults With COVID-19. AJR Am J Roentgenol. 2021;217(4):959-974.
5. Ladopoulos T, Zand R, Shahjouei S, et al. COVID-19: Neuroimaging Features of a Pandemic. J Neuroimaging. 2021;31(2):228-243.
6. Parsons N, Outsikas A, Parish A, et al. Modelling the Anatomic Distribution of Neurologic Events in Patients with COVID-19: A Systematic Review of MRI Findings. AJNR Am J Neuroradiol. 2021;42(7):1190-1195.
7. Katal S, Balakrishnan S, Gholamrezanezhad A. Neuroimaging and neurologic findings in COVID-19 and other coronavirus infections: A systematic review in 116 patients. J Neuroradiol. 2021;48(1):43-50.
8. Chen B, Chen C, Zheng J, Li R, Xu J. Insights Into Neuroimaging Findings of Patients With Coronavirus Disease 2019 Presenting With Neurological Manifestations. Front Neurol. 2020;11:593520.
9. Chougar L, Shor N, Weiss N, et al. Retrospective Observational Study of Brain MRI Findings in Patients with Acute SARS-CoV-2 Infection and Neurologic Manifestations. Radiology. 2020;297(3):E313-E323.
10. Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study. Neurology. 2020;95(13):e1868-e1882.
11. Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI Findings in Patients in the Intensive Care Unit with COVID-19 Infection. Radiology. 2020;297(1):E232-E235.
12. Douaud G, Lee S, Alfaro-Almagro F, et al. Brain imaging before and after COVID-19 in UK Biobank. medRxiv. Published online August 18, 2021.
13. Newcombe VFJ, Spindler LRB, Das T, et al. Neuroanatomical substrates of generalized brain dysfunction in COVID-19. Intensive Care Med. 2021;47(1):116-118.
14. Lu Y, Li X, Geng D, et al. Cerebral Micro-Structural Changes in COVID-19 Patients - An MRI-based 3-month Follow-up Study. EClinicalMedicine. 2020;25:100484.
15. Benedetti F, Palladini M, Paolini M, et al. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: A multimodal magnetic resonance imaging study. Brain Behav Immun Health. 2021;18:100387.
16. Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020;206:116189.
17. Oishi K, Faria A, Jiang H, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: Application to normal elderly and Alzheimer’s disease participants. NeuroImage. 2009;46(2):486-499.
18. Qi X, Arfanakis K. Regionconnect: Rapidly extracting standardized brain connectivity information in voxel-wise neuroimaging studies. Neuroimage. 2021;225:117462.
19. De Santis S, Canals S. Non-invasive MRI windows to neuroinflammation. Neuroscience. 2019;403:1-3.
20. Garcia-Hernandez R, Cerdán Cerdá A, Carpena AT, et al. Mapping microglia and astrocytes activation in vivo using diffusion MRI. bioRxiv. Published online 2021.
21. DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139 Suppl 2(Suppl 2):136-153.
Figures