0035
Pathological Validation of Multiple Sclerosis Lesion Rims on Phase and Quantitative Susceptibility Mapping (QSM) images1Department of Radiology, Weill Cornell Medicine, New York, NY, United States, 2Department of Neurology, Weill Cornell Medicine, New York, NY, United States, 3Department of Neurology, TN Valley HealthCare VA Medical System, Nashville, TN, United States, 44Department of Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 5Department of Neurology, Yale School of Medicine, New Haven, CT, United States
Synopsis
Keywords: Multiple Sclerosis, Quantitative Susceptibility mapping, Phase, iron
Multiple Sclerosis (MS) is an autoimmune disorder characterized by focal inflammatory demyelination. Chronic MS lesions can contain chronically activated, iron-laden microglia and macrophages. By comparing rim status on quantitative susceptibility mapping (QSM) and phase imaging with histopathology that identifies iron, we demonstrate that QSM is a more reliable indicator of iron status than phase. QSM is a valuable clinical tool to identify iron positive smoldering lesions not visible using conventional MRI techniques.Introduction
MS is a chronic inflammatory disease of the central nervous system characterized by formation of inflammatory-demyelinating lesions. Acute inflammatory lesions have a compromised blood brain barrier (BBB) and can therefore be visualized with contrast-enhanced T1-weighted imaging1. Some acute lesions evolve into chronic inflammatory lesion (CAL) featured by iron accumulation in microglia and macrophages2-7 at the perimeter of the lesion core. Due to the presence of iron, non-invasive MR imaging techniques such as QSM and phase MRI are attractive methods for monitoring CAL. Questions remain how these two methods compare in terms of sensitivity and specificity to CAL along with inter-rater variability. Answering this question is of clinical relevance as iron positive rims relate to disability progression and poor clinical outcomes3, 8, 9.Methods
Fifteen formalin-fixed coronal brain slabs from 14 MS patients were obtained from the Rocky Mountain MS Center Tissue Bank. MS brain slabs were embedded in 1% agarose and scanned on 3T clinical MRI scanners (GE Healthcare, Milwaukee, WI; SIEMENS, Erlangen) using the product head coil. The typical imaging protocol consisted of 2D T2‐weighted fast spin echo sequence (voxel size = 0.3 × 0.3 × 0.3 mm3, TE = 57 msec, TR = 6.8 sec, readout bandwidth (rBW) = 195 Hz/pixel, echo train length = 23, number of signal averaging = 6) and 3D multi‐echo gradient echo (GRE) sequence (voxel size = 0.3 x 0.3 x 0.3 mm3, first TE = 4.5 msec, ΔTE = 7.8 msec, TR = 50 msec, flip angle = 15 degrees, rBW = 260 Hz/pixel) for QSM and phase maps. QSM images were reconstructed using morphology enabled dipole inversion.Blinded to lesion histology, three readers (IK, FB, and SAG) classified as rim positive or negative on QSM and Phase 32 white matter hyperintense lesions detected on T2-weighted fluid attenuated inversion recovery. Lesions were classified as rim positive or negative if two or more readers agreed.
Following MRI, lesions of interest were excised, embedded in paraffin, and cut into 5μm sections. Sections were further processed for histology and then incubated overnight with a primary antibody against myelin basic protein (MBP, Dako A0623, 1:500) and a biotinylated secondary antibody and avidin/biotin staining kit with diaminobenzidine (DAB) as the chromogen (Vector Laboratories ABC Elite Kit and DAB Kit). To detect ferric iron, slides were immersed in 4% ferrocyanide/4% hydrochloric acid for 30 minutes in the dark. Staining was enhanced through incubation with DAB for 30 minutes at room temperature. After staining, all sections were rinsed, dehydrated, cover-slipped, and digitized using a Mirax digital slide scanner (Molecular Cytology Core Facility, Memorial Sloan Kettering Cancer Center).
Independent of the readers for the MRI study, two additional readers (KMG and DP) confirmed the presence of an MS lesion using MBP staining, and then classified a lesion as iron+ (iron present at the lesion periphery) or iron- (no iron present at the lesion periphery) using Perls’ stain.
Results
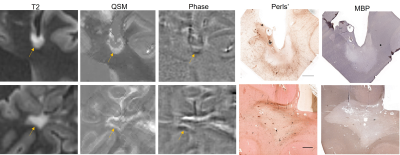
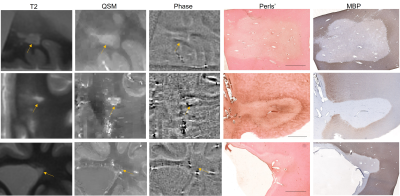
All 32 lesions had complete or near complete loss of myelin in the core, confirming their classification as MS white matter lesions. Of these 32 lesions, 9 were classified as rim positive on both QSM and Phase (QSM+/Phase+), 7 were rim negative on QSM but rim positive on Phase (QSM-/Phase+), 1 was rim positive on QSM but negative on Phase (QSM+/Phase-), and 15 were rim negative on both QSM and Phase (QSM-/Phase-). The Fleiss coefficient for all three raters was moderate (0.57) for QSM rim status and slight (0.33) for Phase rim status.All 10 QSM+ lesions had iron+ rims on histology, while 10 out of 16 phase+ lesions were iron+ lesions on Perls’. One QSM- lesion had an iron+ rim, and the remaining 21 QSM- lesions were iron-. Fifteen phase- lesions were also iron-, but 1 phase- lesion was iron+. Figure 1 shows a QSM+/Phase+ lesion (top row) and a QSM+/Phase- lesion (bottom row); both lesions contain iron at the rim. Figure 2 shows a QSM-/Phase+ lesion that is iron- (top row), a QSM-/Phase+ lesion that is iron+, and a QSM-/Phase- lesion (bottom row) that is iron-. Using the presence of iron on histology as the ground truth, average accuracy for all 3 reviewers was 89.6 ± 4.8% for QSM rim status and 71.9 ± 13.6% for phase rim status. The sensitivity and specificity for QSM rim status were 0.91 and 1.0, respectively. The sensitivity and specificity for phase rim status were 0.91 and 0.71, respectively.
Discussion
Our work demonstrates that lesions with high susceptibility rims, e.g., QSM+ lesions, are more accurate indicators of the presence of iron. This is likely to be of clinical relevance given that iron is present within pro-inflammatory microglia and macrophages, and iron positive rim lesions can slowly expand over time; this expansion can predict disability progression and poor clinical outcomes.Conclusion
Our results indicate that for postmortem imaging QSM is a more reliable indicator than phase of an iron positive rim.Acknowledgements
This work was supported in part by National Institute of Neurological Disorders and Stroke grants R01NS090464, R01NS102667, and R01NS105144; National Multiple Sclerosis Society grant RR‐1602‐07671; NIH Office of the Director grant S10OD021782.References
1. Eskreis-Winkler S, Zhang Y, Zhang J, et al. The clinical utility of QSM: disease diagnosis, medical management, and surgical planning. NMR Biomed. Apr 2017;30(4)doi:10.1002/nbm.3668 2. Mehta V, Pei W, Yang G, et al. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS One. 2013;8(3):e57573. doi:10.1371/journal.pone.0057573
3. Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. Jan 2017;133(1):25-42. doi:10.1007/s00401-016-1636-z
4. Wisnieff C, Ramanan S, Olesik J, Gauthier S, Wang Y, Pitt D. Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: Interpreting positive susceptibility and the presence of iron. Magn Reson Med. Aug 2015;74(2):564-70. doi:10.1002/mrm.25420
5. Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. Jul 1 2016;126(7):2597-609. doi:10.1172/JCI86198 6. Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. Dec 2011;134(Pt 12):3602-15. doi:10.1093/brain/awr278 7. Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. Apr 2021;8(4):877-886. doi:10.1002/acn3.51338 8. Elliott C, Belachew S, Wolinsky JS, et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain. Sep 1 2019;142(9):2787-2799. doi:10.1093/brain/awz212
9. Absinta
M, Sati P, Masuzzo F, et al. Association of Chronic Active Multiple Sclerosis
Lesions With Disability In Vivo. JAMA
Neurol. Dec 1 2019;76(12):1474-1483. doi:10.1001/jamaneurol.2019.2399
Figures