0032
Regional Changes in Brain Viscoelasticity in Multiple Sclerosis Assessed with Three-Dimensional MR Elastography1Department of Radiology, the Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China, 2Department of Radiology, Mayo Clinic, Rochester, MN, United States, 3Department of Neurology, the Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
Synopsis
Keywords: Multiple Sclerosis, Elastography
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system. MR Elastography (MRE) can quantitatively assess biomechanical tissue properties in vivo noninvasively. We investigated the potential value of MRE to reveal changes in tissue viscoelasticity in regions and the whole brain in MS patients and to analyze the relevance to clinical manifestations. Our results suggest that the damping ratio and loss modulus are promising quantitative biomarkers for evaluating tissue damage in MS.Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system leading to the breakdown of the blood-brain barrier, multifocal inflammation, oligodendrocyte loss, demyelination, reactive gliosis and axonal degeneration1. Magnetic resonance elastography (MRE) can quantifying tissue mechanical properties in a noninvasive way and may be sensitive to these pathological processes2-4. It has been reported that whole-brain viscoelasticity and stiffness decreased in subjects with MS5, 6 and the range of stiffness changes in white matter lesions due to MS is within the normal range of white matter variability7. The aim of this study is to further assess changes in 3D MRE-based measurements of brain viscoelasticity in MS and to examine relationships between brain viscoelasticity and clinical data in MS patients.Methods
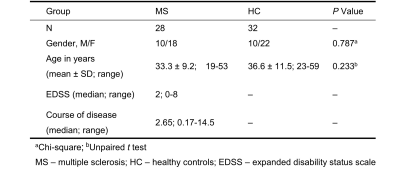
Following ethics committee approval with a waived informed consent requirement, 28 patients with MS and 32 healthy volunteers underwent conventional MRI and brain MRE scans with 60-Hz vibration frequency using a pillow-like MRE driver developed at Mayo Clinic. Clinical parameters including the expanded disability status scale (EDSS) and disease course (defined as years since diagnosis) were recorded. The acquisition parameters for 3D MRE were as follows: TR/TE = 2000/62 ms, FOV = 24×24 cm; acquisition matrix = 96×96; number of excitations = 1; Bandwidth = 250 kHz; slices thickness = 3 mm with gap = 0 mm. The success of MRE was defined as visually detectable wave propagation in the whole brain. ROI-based stiffness, storage modulus and loss modulus measurements were obtained, and ROIs were drawn with reference to the MR images. The damping ratio (the loss modulus divided by two times the storage modulus) was also calculated. EDSS scores were obtained by two experienced neurologists for each patient in consensus. We compared the MRE parameters between the two groups using an unpaired t-test and among the brain regions using multiple t test. The MRE parameters were also compared to the disease course and EDSS score with Spearman correlation. Statistical significance was defined as P<0.05.Results
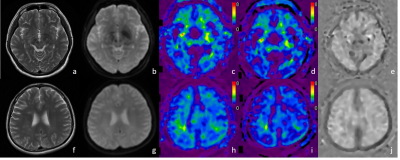
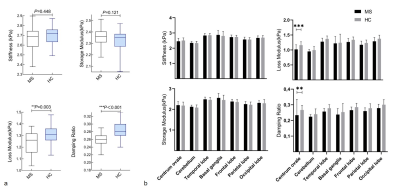
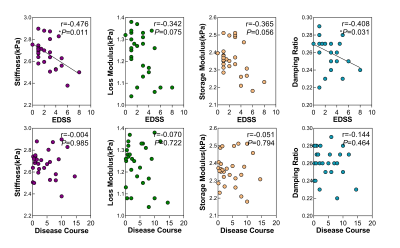
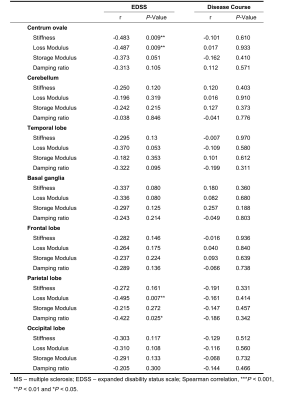
Demographic data and clinical characteristics are shown in Table 1. MRE images for 2 subjects are shown in Figure 1.In this study, compared to the health controls, the mean loss modulus (1.23 ± 0.10 kPa vs. 1.31 ± 0.08 kPa, P=0.003) and the mean damping ratio (0.26 ± 0.02 vs. 0.28 ± 0.02, P<0.001) of the whole brain were significantly decreased in MS(Figure 2a). The results of the brain regions viscoelasticity in patients with MS and healthy controls are shown in Figure 2a. The mean loss modulus and the mean damping ratio of the centrum ovales were significantly decreased in MS patients while there were no significant differences in other regions (cerebellum, temporal lobe, basal ganglia, frontal lobe, parietal lobe and occipital lobe) compared to the health controls (Figure 2b). There was a downward trend for loss modulus and storage modulus over the whole brain with increasing EDSS (loss modulus: r=-0.342, P=0.075; storage modulus: r=-0.365, P=0.056), but no statistical significances (Figure 3). Both damping ratio and stiffness had a significant negative correlation with EDSS score (stiffness: r=-0.476, P=0.011; damping ratio: r=-0.408, P=0.031), while no correlations were found between the MRE parameters and the disease course(Figure 3). In Table 2, there were negative correlations between some MRE parameters (stiffness and loss modulus of the centrum ovale, loss modulus and damping ratio of parietal lobe) and EDSS scores in MS, but no correlation with increased disease course was observed.Discussion
Some studies have reported that global brain stiffness is reduced in MS5, 6. Our results showed significantly decreased damping ratio and loss modulus over the whole brain and the centrum ovale in MS patients, while the stiffness and storage modulus did not decrease in this study. The results suggest that loss modulus and damping ratio are promising noninvasive biomarkers to assess brain inflammation8 damage due to MS and the centrum ovale is one of the most affected sites in brain. There were negative correlations between MRE parameters (damping ratio and stiffness in the whole brain, stiffness and loss modulus in centrum ovale and damping ratio and loss modulus in parietal lobe) and EDSS scores, suggesting the potential of MRE to be used to assess the clinical severity. The results provide motivation for larger patient studies.Conclusion
Damping ratio and loss modulus were decreased in the centrum ovale and the whole brain of MS patients and 3D MRE may be a potential method to evaluate brain tissue damage in MS.Acknowledgements
National Natural Science Foundation of China grant 91959118 (JW) and 82271973(JW), Key Research and Development Program of Guangdong Province 2019B020235002 (JW), Guangdong Basic and Applied Basic Research Foundation, 2021A1515010582 (JW), SKY Radiology Department International Medical Research Foundation of China Z-2014-07-2101 (JW) and Clinical Research Foundation of the 3rd Affiliated Hospital of Sun Yat-sen University YHJH201901 (JW).References
1. Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 2008; 31:247-269.
2. Schregel K, Wuerfel E, Garteiser P, et al. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc Natl Acad Sci U S A 2012; 109:6650-6655.
3. Riek K, Millward JM, Hamann I, et al. Magnetic resonance elastography reveals altered brain viscoelasticity in experimental autoimmune encephalomyelitis. Neuroimage Clin 2012; 1:81-90.
4. Fehlner A, Behrens JR, Streitberger KJ, et al. Higher-resolution MR elastography reveals early mechanical signatures of neuroinflammation in patients with clinically isolated syndrome. J Magn Reson Imaging 2016; 44:51-58.
5. Wuerfel J, Paul F, Beierbach B, et al. MR-elastography reveals degradation of tissue integrity in multiple sclerosis. Neuroimage 2010; 49:2520-2525.
6. Streitberger KJ, Sack I, Krefting D, et al. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. Plos One 2012; 7: e29888.
7. Herthum H, Hetzer S, Scheel M, et al. In vivo stiffness of multiple sclerosis lesions is similar to that of normal-appearing white matter. Acta Biomaterialia 2022; 138:410-421.
8. Yin M, Glaser KJ, Manduca A, et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology 2017; 284:694-705.
Figures