0029
Disruption to normal excitatory and inhibitory function within the medial prefrontal cortex in patients with IBD1Second Clinical School, Lanzhou University, Lanzhou, China, 2Department of Magnetic Resonance, Lanzhou University Second Hospital, Lanzhou, China, 3Gansu Province Clinical Research Center for Functional and Molecular Imaging, Lanzhou University Second Hospital, Lanzhou, China, 4Department of clinical science, Philips Healthcare, Xi’an, China
Synopsis
Keywords: Neuroinflammation, fMRI
The etiology of IBD complicated with anxiety, depression and other psychological comorbidities is still unclear. The purpose of this study was to quantify neurotransmitter (GABA and Glx) in the medial prefrontal cortex (mPFC) of IBD patients with MEGA-PRESS spectroscopy and explore its relationship with clinical scores. We found that mPFC GABA+ and Glx concentration were significantly decreased in IBD patients and were associated with abdominal pain and depressive symptoms separately, suggesting psychological comorbidities in IBD patients were related to mPFC neurotransmitter dysregulation. It provides a new explanation for the neuropsychological mechanism of IBD in the development of the disease.
Introduction
Inflammatory bowel disease (IBD) is a kind of immune mediated gastrointestinal inflammatory disease [1]. The incidence rate is increasing year by year, affecting the health of about 7 million people around the world, and has become an important global health burden. The two main forms of IBD are ulcerative colitis (UC) and Crohn's disease (CD) [2]. The hypothetical cause of anxiety and depression is systemic low-grade neuroinflammation caused by IBD patients [3-6], but the mechanism is still unclear. Gamma-aminobutyric acid (GABA), as the main inhibitory neurotransmitter in human brain, has an important regulatory effect on brain function [7]. In vivo magnetic resonance spectroscopy (MRS) imaging can non-invasively detect the content of metabolites in human brain tissue [8]. This study aims to explore the mechanism of common psychological symptoms in IBD patients by examining changes in GABA and glutamate + glutamine (Glx) concentrations in mPFC in IBD patients and their relationship with pain and psychological comorbidities.Materials and Methods
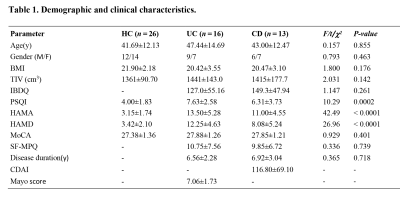
In this prospective study, 26 IBD patients (16 UC, 13 CD) and 26 age/sex-matched health controls were included. The Human Research Ethics Committee of Lanzhou University Second Hospital approved the study, and all participants gave informed written consent in accordance with the Helsinki Declaration. Data were collected using a 3.0 T MR scanner (Ingenia CX, Philips Healthcare, Netherlands) with 32 channel head coils. The edit MRS was obtained from voxels (30 × 30 × 20 mm3) in the medial prefrontal cortex (mPFC). MEGA-PRESS parameters are as follows: [TR]=2000 ms, [TE]=68 ms, Gaussian editing pulses at 1.89 parts per million (ppm) of the proton frequency (edit-ON) and 7.46 ppm (edit-OFF), 288 averages, 1024 acquisition points and VAPOR water suppression. MRS data were analyzed using Gannet 3.0.(http://www.gabamrs.com/) [9]. The measured GABA concentration using MEGA-PRESS protocol commonly referred to as GABA+ with the contribution from macromolecules. The contents of GABA+ and Glx in gray matter were evaluated by segmenting gray matter, white matter, and cerebrospinal fluid with spm12 toolbox.Statistical analysis was performed using SPSS version 25.0 (IBM). The comparison of group differences was performed using a one-way ANOVA. Significant correlations between parameter data variables were examined by a two-tailed Pearson correlation. Subgroup analysis of UC and CD was performed. In addition, we used logistic regression to assess whether GABA +/Glx concentrations were able to identify IBD patients from normal individuals. A significance level of p < .05 was set.
Results
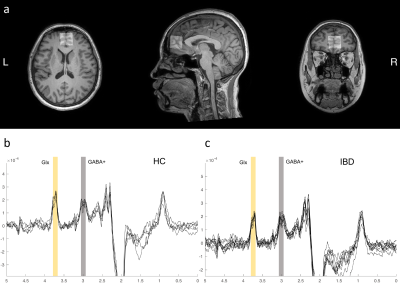
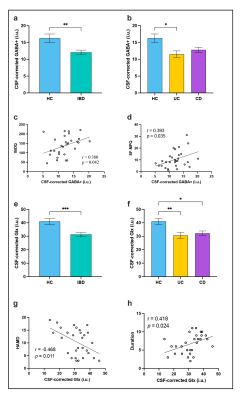
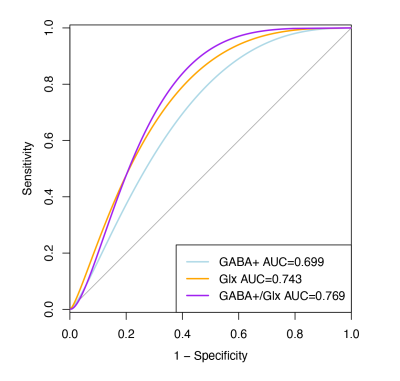
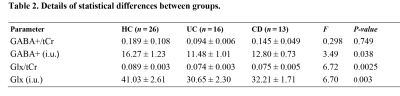
The results of clinical data are shown in Table 1. Volume-of-interest (VOI) of MRS was placed at mPFC, as shown in Fig. 1a. Mean and standard deviation of GM/WM/CSF were 58.1 ± 5%, 24.3 ± 6%, and 17.6 ± 4%, respectively. A typical GABA +/Glx spectra obtained from mPFC were shown in Fig. 1b, c. Table 2 shows the details of statistical differences between groups. There was no statistical difference between groups for GABA+/tCr. Compared with HC, the GABA+ concentration in mPFC decreased significantly in IBD subjects (shown in Fig. 2a and Fig. 2b). In subjects with IBD, the concentration of GABA+ was positively correlated with IBDQ (r = 0.380, p = 0.042) and SF-MPQ (r = 0.393, p = 0.035). At the same time, we compared Glx/tCr between groups and found that there were significantly different among them. Compared with HC, the Glx concentration decreased significantly (shown in Fig. 2e and Fig. 2f.). The Glx concentration was negatively correlated with HAMD score (r = -0.468, p = 0.011), and significantly positively correlated with disease duration (r = 0.418, p = 0.024). Receiver operating characteristic (ROC) curve analysis was performed to compare the ability of MRS to differentiate IBD from HCs. Overall, the classification performance was better based on GABA+ and Glx (Accuracy = 76.36 %, AUC = 0.763). Compared to GABA+ (Accuracy= 69.09%, AUC=0.699), Glx obtained better classification accuracy (Accuracy = 72.73%, AUC=0.743). Fig 3 shows the ROC curves and the corresponding AUC.Discussion
In this study, we investigated the neurochemical transmitters’ abnormalities of mPFC in patients with IBD. The main findings were as follows: First, the levels of mPFC Glx and GABA+ in IBD patients decreased significantly, which was related to abdominal pain and depressive symptoms. These findings suggest that the hypothesis that emotional disorders in IBD patients are related to the imbalance of mPFC neurotransmitters. Secondly, we found that the classification model based on MRS can effectively distinguish IBD and HC, indicating that MRS is a potential biomarker. Use quantitative magnetic resonance spectroscopy to solve problems that may be related to symptoms [10, 11]. Above results are consistent with previous studies on chronic pain [12]. It indicates that the change of the level of GABA+ and Glx in mPFC is related to the intensity of pain and the patient's subjective experience of disease.Conclusion
In conclusion, this study revealed a decrease trend in GABA+ and Glx levels in mPFC associated with clinical symptoms of IBD. These findings provide us a new insight into the neural mechanisms related to IBD. In the future, the results of intestinal microbiome and metabolome research will help us understand the potential rules of brain gut interaction in IBD patients. It also provides a broad prospect for us to find a treatment plan.Acknowledgements
We are grateful to all the participants for their cooperation and patience.References
1. MacDonald, S., et al. (2022). Stakeholder Perspectives on Access to IBD Care: Proceedings From a National IBD Access Summit. J Can Assoc Gastroenterol, 5(4), 153-160. https://doi.org/10.1093/jcag/gwab048
2. Sands, B.E. (2015). Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology, 149(5), 1275-1285.e2. https://doi.org/10.1053/j.gastro.2015.07.003
3. Chassaing, B., et al. (2014). AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut, 63(7), 1069-80. https://doi.org/10.1136/gutjnl-2013-304909
4. Craig, C.F., et al. (2022). Neuroinflammation as an etiological trigger for depression comorbid with inflammatory bowel disease. J Neuroinflammation, 19(1), 4. https://doi.org/10.1186/s12974-021-02354-1 5. Greuter, T., et al. (2018). Low serum zinc levels predict presence of depression symptoms, but not overall disease outcome, regardless of ATG16L1 genotype in Crohn's disease patients. Therap Adv Gastroenterol, 11, 1756283x18757715. https://doi.org/10.1177/1756283x18757715
6. Rhie, S.J., E.Y. Jung, and I. Shim. (2020). The role of neuroinflammation on pathogenesis of affective disorders. J Exerc Rehabil, 16(1), 2-9. https://doi.org/10.12965/jer.2040016.008
7. Wang, Q. and Y. Dwivedi. (2021). Advances in novel molecular targets for antidepressants. Prog Neuropsychopharmacol Biol Psychiatry, 104, 110041. https://doi.org/10.1016/j.pnpbp.2020.110041
8. Menke, R.A., et al. (2017). Neuroimaging Endpoints in Amyotrophic Lateral Sclerosis. Neurotherapeutics, 14(1), 11-23. https://doi.org/10.1007/s13311-016-0484-9
9. Edden, R.A., et al. (2014). Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging, 40(6), 1445-52. https://doi.org/10.1002/jmri.24478
10. Gordon-Lipkin, E., et al. (2018). ST3GAL5-Related Disorders: A Deficiency in Ganglioside Metabolism and a Genetic Cause of Intellectual Disability and Choreoathetosis. J Child Neurol, 33(13), 825-831. https://doi.org/10.1177/0883073818791099
11. Gonzales, M.M., et al. (2013). Aerobic fitness and the brain: increased N-acetyl-aspartate and choline concentrations in endurance-trained middle-aged adults. Brain Topogr, 26(1), 126-34. https://doi.org/10.1007/s10548-012-0248-8
12. Lee, J., et al. (2021). 3D magnetic resonance spectroscopic imaging reveals links between brain metabolites and multidimensional pain features in fibromyalgia. Eur J Pain, 25(9), 2050-2064. https://doi.org/10.1002/ejp.1820
Figures