0022
Estimation of pH values and magnesium ion content in vivo using a chemical shift dictionary for 31P MRSI at UHF1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 3Division of Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Faculty of Medicine, University of Heidelberg, Heidelberg, Germany, 5Division of Neuroradiology, University Hospital Bonn, Bonn, Germany
Synopsis
Keywords: Spectroscopy, Non-Proton, 31P, phosphorus, pH
31P MRSI enables non-invasive mapping of pH value and magnesium content in vivo, and is conventionally done by calibration equations, like the modified Henderson-Hasselbalch equation, relating 31P chemical shifts to physiological parameters. The reliability of this approach might be hampered when applied to diseased tissue, like cancer, where the chemical conditions are altered. Recently, we proposed a chemical shift dictionary for 31P MRSI to potentially enable a condition-independent estimation of pH value and magnesium content. In this study, the applicability of this approach to in vivo data is tested, and its potential for further research identified.Introduction
31P MRSI enables non-invasive imaging of pH values and magnesium ion content in living tissues1-3, employing the chemical shift changes of inorganic phosphate (Pi) and Adenosine-5’-Triphosphate (ATP). The chemical shifts are related to biochemical parameters via calibration equations, like the Henderson-Hasselbalch equation (HHE) in the case of pH4. However, therein required constants, e.g. pKA, are typically only characterized under physiological conditions. This poses a particular challenge in pathological conditions, e.g. in tumor tissue, where chemical conditions might change manifold, hence potentially hampering reliability of the employed equations. Recently, we proposed a dictionary-based approach potentially enabling the estimation of pH and magnesium ion concentration under varying chemical conditions, i.e. ionic strength, which might provide a valuable alternative to characterize the tissue’s microenvironment5. The purpose of this study was to test the applicability of this dictionary-based approach for in vivo 31P MRSI data, and to identify its potential value for biomedical research.Methods
The implemented dictionary is based on the chemical shifts of $$$\gamma$$$- and $$$\beta$$$-ATP obtained from 31P FID measurements at 9.4T (Bruker) in 114 model solutions prepared with different (pH, R = [Mg2+] /[ATP4-], Ion) conditions. The quantity Ion acts as a surrogate for the true ionic strength scaled with an arbitrary factor. To extend the dictionary, the chemical shifts were interpolated using a multidimensional model function based on the Hill equation, yielding a final number of 426,951 entries. The dictionary search algorithm assigns output values for (pH, R, and Ion) for a given chemical shift combination based on combined probability density functions for the interpolation model, as described in detail in5. The algorithm was applied to in vivo 3D 31P MRSI datasets measured at 7T (Magnetom 7T, Siemens), comprising data from lower leg muscles of 3 healthy volunteers3, and data from brain tissue of 3 patients with glioblastoma2. For a description of the 31P MRSI data acquisition and quantification, the reader is referred to1-3. Locally quantified chemical shifts of $$$\gamma$$$- and $$$\beta$$$-ATP were fed into the dictionary algorithm to yield voxelwise output triples (pHDict, RDict, IonDict), resulting in three different 3D maps for each volunteer and patient. For comparison, conventional maps for pH and the free magnesium ion concentration [Mg2+free] were calculated with the modified HHE with constants from4 for pH, and the approach by Golding and Golding6 for magnesium. Regions-of-interest (ROIs) covering three different muscle groups (muscle data), and ROIs covering the whole tumor and white matter (brain data), were defined on morphological images.Results
All in vivo datasets showed regional variations in the combination of the quantified chemical shifts of $$$\gamma$$$- and $$$\beta$$$-ATP. Applied to the in vivo datasets, the dictionary algorithm successfully assigned output values (pHDict, RDict, IonDict) in 98% (muscle) and 90% (brain) of all tissue voxels, showing that the dictionary space well covers the chemical shift combinations observed in vivo. Representative 3D maps of the output triples (pHDict, RDict, IonDict) in the lower leg muscles of one healthy volunteer (Figure 1) and in the brain of one patient with glioblastoma (Figure 2) are shown in comparison to the conventionally calculated pH and [Mg2+free] maps. The ROI analyses are presented for all volunteers and patients (Figures 3 and 4), comparing the median values between dictionary and conventional maps. The dictionary maps resemble the morphological features of the conventional maps, but have different absolute output values. In the magnesium maps, the trends of high and low values are the same for both maps (RDict and Mgconv), whereas a stronger variation is observed in the pHDict maps, which is in accordance with a reversed variation in the IonDict map. For the leg muscle datasets, the maps and the ROI analysis yielded the same patterns and trends for all volunteers. For the glioblastoma data, the trends in the pHDict and IonDict maps differ between patients.Discussion
While for the muscle data the results are comparable between all volunteers (only muscle tissue being present), the situation is more complex in the brain, where brain parenchyma, muscle tissue (voxel bleed) and tumors with varying biochemistry may interfere, yielding a larger variation in chemical shift combinations. The stronger fluctuations in the dictionary maps of the patients (Figure 4) could be explained by strong differences in the ionic strength, which might be expected for heterogeneous glioma tissue. The resemblance of the IonDict map with the pHconv map in the muscle datasets might be a hint towards a change in the pKA value in some tissue areas, leading to higher pHconv values calculated via the HHE, as pKA changes with ionic strength. However, the current version of the dictionary is so far not sensitive enough to ionic strength (coarse spacing of Ion entries), which needs to be refined in future work.Conclusion
The proposed dictionary algorithm successfully assigns plausible biochemical parameters to in vivo 31P MRSI data, and might provide an alternative to the conventional approaches to obtain pH values and magnesium ion content in the future. The lack of necessity to calibrate physical quantities, e.g. pKA, makes the application to tissues with a priori unknown chemical microenvironment like tumors particularly interesting. Using this approach, novel knowledge about the tumor microenvironment could be obtained in the future.Acknowledgements
No acknowledgement found.References
1. Korzowski A, Weckesser N, Franke VL, Breitling J, Goerke S, Schlemmer HP, Ladd ME, Bachert P, Paech D. Mapping an Extended Metabolic Profile of Gliomas Using High-Resolution 31P MRSI at 7T. Front Neurol. 2021 Dec 23;12:735071. doi:10.3389/fneur.2021.735071
2. Korzowski, A, Weinfurtner, N, Mueller, S, et al. Volumetric mapping of intra- and extracellular pH in the human brain using 31P MRSI at 7T. Magn Reson Med. 2020; 84: 1707– 1723. https://doi.org/10.1002/mrm.28255
3. Franke, VL, Breitling, J, Ladd, ME, Bachert, P, Korzowski, A. 31P MRSI at 7 T enables high-resolution volumetric mapping of the intracellular magnesium ion content in human lower leg muscles. Magn Reson Med. 2022; 88: 511- 523. doi:10.1002/mrm.29231
4. de Graaf RA. In Vivo NMR Spectroscopy: Principles and Techniques: 2nd Edition.; 2007. doi: 10.1002/9780470512968.
5. Franke, VL, Breitling, J, Bangert, R, Ladd, ME, Bachert, P, Korzowski, A. A dictionary-based approach for the determination of pH values using 31P MRS. Proceedings of the ISMRM, London, England, UK, 2022. Abstract number #1364.
6. Golding EM, Golding RM. Interpretation of 31P MRS Spectra in Determining Intracellular Free Magnesium and Potassium Ion Concentrations.; 1995.
Figures
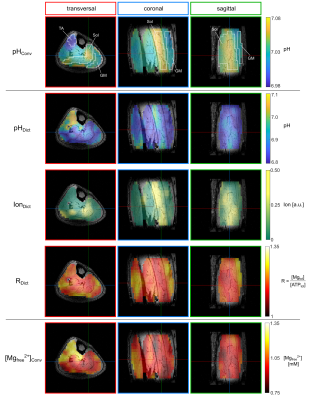
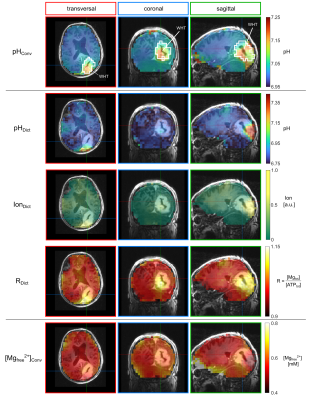
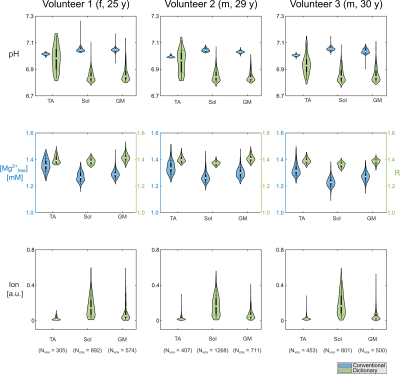
Figure 3: Regions-of-interest analysis represented as violin plots for pH value, magnesium content and ionic strength (with arbitrary scaling factor) for the muscles tibialis anterior (TA), soleus (Sol), and gastrocnemius medialis (GM) for all three volunteers. Results from the dictionary estimation (green) are compared to conventionally calculated pH and [Mg2+free] maps (blue). For the ionic strength, only values from the dictionary estimation are available. The magnesium content from the dictionary is given as R = [Mgtotal] / [ATPtotal].
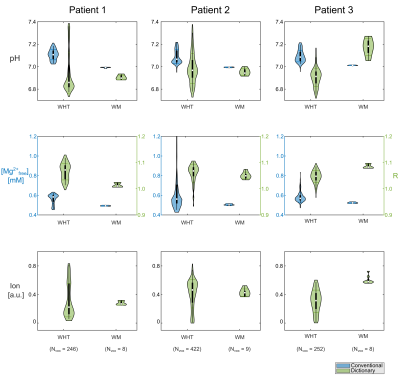
Figure 4: Regions-of-interest (ROI) analysis represented as violin plots for pH value, magnesium content and ionic strength (with arbitrary scaling factor) for the whole tumor (WHT) and the white matter ROI (WM) for all patients. Results from the dictionary estimation (green) are compared to conventionally calculated pH and [Mg2+free] maps (blue). For ionic strength, only values from the dictionary estimation are available. The magnesium content from the dictionary is given as R = [Mgtotal]/[ATPtotal]. Note that in patient 3 no consistent WM ROI could be defined contralaterally