0021
Whole-brain bi-exponential 23Na-MRI T2* mapping at 7T with a 32-channel phased array receiver coil1NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, United Kingdom, 2Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 3Department of Radiology, Imperial College Healthcare NHS Trust, London, United Kingdom, 4Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 5London Collaborative Ultra high field System (LoCUS), London, United Kingdom, 6Children's Neurosciences, Evelina London Children's Hospital at Guy's and St Thomas' NHS Foundation Trust, London, United Kingdom, 7Guys and St Thomas’ NHS Foundation Trust, King's College London, London, United Kingdom, 8Department of Biomedical Engineering, The University of Melbourne, Melbourne, Australia, 9Melbourne Brain Imaging Unit, The University of Melbourne, Melbourne, Australia, 10Melbourne Neuropsychiatry Centre, The University of Melbourne, Parkville, Victoria, Australia, 11Quantitative Imaging Group, Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 12Department of Brain & Behavioural Sciences, University of Pavia, Pavia, Italy, 13Brain Connectivity Centre Research Department, IRCCS Mondino Foundation, Pavia, Italy
Synopsis
Keywords: Non-Proton, Relaxometry, Sodium, T2* mapping
In vivo 23Na-MRI benefits greatly from SNR improvements at ultrahigh fields and with multi-channel receivers. Here, we report a pipeline for multi-echo radial imaging of 23Na (MERINA) at 7T, using a 32-channel receiver coil. This involves correction of image artifacts induced by gradient imperfections, followed by channel combination to maintain Rician noise distributions in combined magnitude images. This led to improved T2* mapping with a fixed-component bi-exponential signal model. T2* values (e.g. T2s*=4.6±0.9ms, T2l*=28.3±2.8ms in cerebral white matter) agree with reports in literature. Future work will involve correcting B0 inhomogeneity effects, more sophisticated signal models and exploring potential clinical applications.Introduction
Sodium magnetic resonance imaging (23Na-MRI) is a promising imaging modality, providing quantitative insight into physiology1. Technological advances in MR-hardware (ultra-high fields and multi-channel receivers2) have increased SNR in 23Na-MRI, enabling novel applications of quantitative methods like relaxometry3,4, as 23Na relaxation parameters could provide clearer insight into physiology than concentration measures4. A recent example is the Multi-Echo Radial Imaging of NA (MERINA) sequence5 for bi-exponential T2* mapping, as demonstrated at 7T with a single-channel birdcage receiver.As quadrupolar nuclei, 23Na ions in tissue undergo bi-exponential transverse relaxation6. Corresponding signal models are challenging to fit, particularly in low-SNR conditions7. MERINA authors addressed this by parametrising and fitting the Rician probability density. This relied on assumptions that noise in magnitude images is Rician-distributed and spatially invariant. Noise in combined multi-channel images will however be spatially variant, and Rician only for certain combination strategies8. Further, continuous rephasing of echoes in MERINA may accumulate k-space trajectory errors, inducing artifacts in later echoes that compromise T2* maps. Here, we develop a processing pipeline to improve T2* map quality using a 32-channel receiver coil, incorporating optimal channel combination and gradient error correction, to realise MERINA bi-exponential 23Na-MRI T2* mapping in clinically feasible scan times.
Theory
Channel combinationA multi-channel coil with $$$n$$$ receivers acquires $$$n$$$ sensitivity-weighted images, $$$I_c$$$, of the magnetisation, $$$M$$$:
$$I_c=SM. [1]$$
Knowing sensitivities, $$$S$$$, and the noise covariance matrix, $$$\Sigma$$$, a combination operator, $$$C$$$, can compute an SNR-optimal estimate, $$$\hat{M}$$$, according to the weighted least-squares solution9,10:
$$\hat{M}=CI_c=(S^\ast\Sigma^{-1}S)^{-1}S^\ast{}\Sigma^{-1}I_c. [2]$$
The noise in $$$\hat{M}$$$ becomes spatially dependent and can be predicted by11:
$$\sigma^2(x)=\Sigma^{\ast}C\Sigma. [3]$$
Noise will remain complex Gaussian until $$$\lvert\hat{M}\rvert$$$ is calculated, yielding a Rician noise distribution8,11.
Trajectory correction
Gradient imperfections induce blurring and streaking artifacts in radial MRI12. A popular correction approach13 involves acquiring calibration data of a subset of opposed spokes and predicting a shift for all spokes, via the phase of the Fourier transformed cross-correlation.
Here, instead we take advantage of the inherent multi-echo repeats of each radial spoke to characterise and correct trajectory errors.
The trajectory shift for spoke $$$i$$$ of echo $$$TE$$$, $$$K^i_{TE}$$$, with reference to $$$K^i_{1}$$$, can be calculated by finding the peak location of the cross-correlation, $$$G_i$$$, where $$$\mathcal{F}$$$ is the 1D Fourier transform and even-echo $$$K^i_{TE}$$$ are flipped:
$$G_i=\mathcal{F}(\mathcal{F}(K^i_{TE})\mathcal{F}(K^i_{1})^{\ast}). [4]$$
Methods
Acquisition8 paediatric (9-17y, mean=13y) healthy volunteers14 (local ethics HR-18-19-8700) were scanned on a 7T MAGNETOM Terra (Siemens Healthcare, Erlangen, Germany) with a dual-tuned sodium coil (Rapid Biomedical GmbH) with one transmit, two receive (1TX, 1RX/32RX) modes. MERINA 23Na-MRI parameters were5: FOV=200x200x200mm3, matrix=64x64x64, TE=0.35ms, TR=151ms, FA=90º, TRO=2ms, 37 refocused echoes, 2000 spokes, NSA=3 (twice with 32RX, once with 1RX, 5min/TA). Manual shimming was performed before 23Na-MRI.
Trajectory correction
The peak location of the cross-correlation for k-space spokes $$$K^i_{2}$$$ to $$$K^i_{15}$$$ (Eq. 4) was found by quadratic interpolation, yielding a sub-resolution shift that was applied to remaining echoes. Echoes 0,1 were realigned by interpolating the location of the central k-space maximum. Shifts were computed for 1RX data and applied to 32RX data.
Image reconstruction
Individual channel images were reconstructed with a previously described pipeline [4], with the addition of the time-efficient FINUFFT library15.
Channel combination
Coil sensitivities were estimated using the 1RX image16 and smoothed (Gaussian kernel, width=3). Noise per channel image was sampled from the signal-free superior-most axial slice, to compute the 32-by-32 noise covariance matrix. Channel images were combined [Eq. 2], also deriving a map of noise variances [Eq. 3] for T2* mapping.
T2* mapping
Bi-exponential T2* mapping was implemented as in5, by maximising the log-likelihood of the Rician probability density, with a fixed-component bi-exponential signal model:
$$S(t,x)=S_0(x)\left[0.6\exp\left(-\frac{t}{T_{2s}^{\ast}(x)}\right)+0.6\exp\left(-\frac{t}{T_{2l}^{\ast}(x)}\right)\right]. [5]$$
Uniquely, here, the Rician probability density was parametrised on a voxel-by-voxel basis, utilising the derived spatial noise variance $$$\sigma^2(x)$$$.
Results
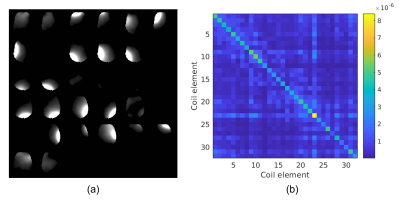
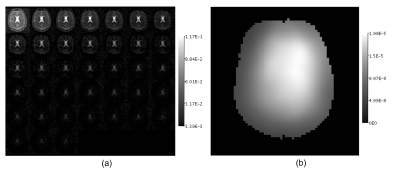
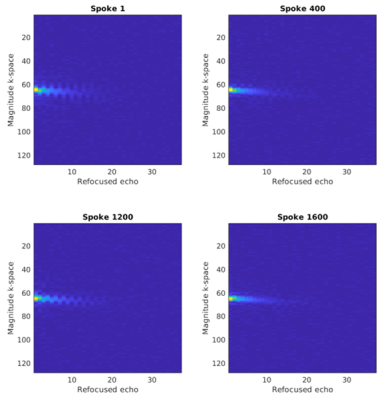
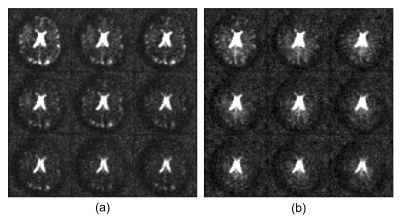
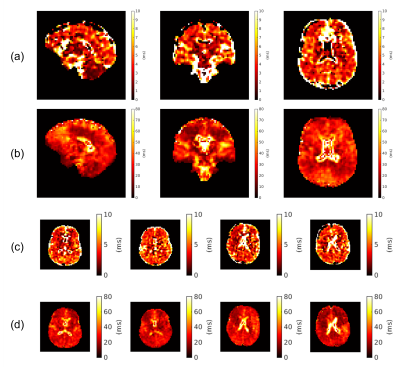
Fig. 1 shows coil sensitivities and the noise covariance matrix used for channel combination. Combined multi-echo images, with a noise map, $$$\sigma^2(x)$$$, are shown in Fig 2. The necessity and effect of k-space trajectory correction are illustrated in Figs. 3, 4. Blurring of CSF signal evident prior to trajectory realignment is supressed. Fig. 5 shows sagittal, coronal, axial T2s* and T2l* of 5 healthy volunteers; averages in cerebral white matter were14 T2s*=4.6±0.9ms, T2l*=28.3±2.8ms.Discussion
Reported values of T2s*, T2l* broadly agree with literature3,4,17,18. Maps show tissue contrast, possibly reflecting underlying differences in physiology. Spatial profiles of the maps appear mostly smooth, apart from some discontinuities in CSF and T2s* regions.Cross-subject spatial modulations in T2l* (Fig. 5) could be driven by susceptibility and field inhomogeneities, which may require B0 corrections or an improved shimming protocol.
The simple trajectory correction implemented is adequate in low-SNR, low-resolution conditions. Nonetheless, following more sophisticated approaches19,20,21 could further improve accuracy of T2* maps.
Lastly, future work will explore advanced signal modelling. Possibilities include: fitting T2* amplitudes (deviations from 60/40 occur in-vivo22); distribution fits with an unrestricted number of components23,24; real image reconstruction25 to avoid fitting the Rician probability density, although temporally consistent phase correction may prove challenging.
Conclusion
We have demonstrated the necessary post-processing adaptations to realise in-vivo bi-exponential 23Na-MRI T2* mapping at 7T with a multi-channel receiver. Next steps will involve further refinement of our pipeline, as well as exploring clinical applications.Acknowledgements
DC and CAMGWK contributed equally.
SR: EPSRC-funded UCL Centre for Doctoral Training in Intelligent, Integrated Imaging in Healthcare (i4health) (EP/S021930/1) and the Department of Health’s NIHR-funded Biomedical Research Centre at University College London Hospitals.
CAMGWK: Horizon2020 (Human Brain Project SGA3, Specific Grant Agreement No. 945539), BRC (#BRC704/CAP/CGW), MRC (#MR/S026088/1), Ataxia UK, MS Society (#77), Wings for Life (#169111). CGWK is a shareholder in Queen Square Analytics Ltd.
DC, PB, SJM, ASD, JVH: Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
ASD: GOSHCC Sparks Grant V4419, King's Health Partners, in part by the Medical Research Council (UK) (grants MR/ K006355/1 and MR/LO11530/1) and Medical Research Council Center for Neurodevelopmental Disorders, King’s College London (MR/N026063/1).
ME: Action Medical Research [GN2835] and the British Paediatric Neurology Association.
PB: This work was supported by a project grant awarded by Action Medical Research [GN2728], a Wellcome Trust Collaboration in science award [WT201526/Z/16/Z].
BSS: Wings for Life (#169111).
We thank Prof. Leigh Johnston, Melbourne Brain Centre Imaging Unit, University of Melbourne, Australia, for her support and contributions.
References
[1] K. R. Thulborn, "Quantitative sodium MR imaging: A review of its evolving role in medicine," NeuroImage, vol. 168, pp. 250-268, 2018.
[2] Y. Qian, T. Zhao, G. C. Wiggins, L. L. Wald, H. Zheng, J. Weimer and F. E. Boada, "Sodium imaging of human brain at 7 T with 15-channel array coil," Magnetic Resonance in Medicine, vol. 68, p. 1807–1814, February 2012.
[3] F. J. Kratzer, S. Flassbeck, S. Schmitter, T. Wilferth, A. W. Magill, B. R. Knowles, T. Platt, P. Bachert, M. E. Ladd and A. M. Nagel, "3D sodium (23Na) magnetic resonance fingerprinting for time-efficient relaxometric mapping," Magnetic Resonance in Medicine, vol. 86, p. 2412–2425, June 2021.
[4] J. M. Lommen, S. Flassbeck, N. G. R. Behl, S. Niesporek, P. Bachert, M. E. Ladd and A. M. Nagel, "Probing the microscopic environment of 23Na ions in brain tissue by MRI: On the accuracy of different sampling schemes for the determination of rapid, biexponential decay at low signal-to-noise ratio," Magnetic Resonance in Medicine, vol. 80, pp. 571-584, 2018.
[5] Y. Blunck, S. Josan, S. W. Taqdees, B. A. Moffat, R. J. Ordidge, J. O. Cleary and L. A. Johnston, "3D-multi-echo radial imaging of 23 Na (3D-MERINA) for time-efficient multi-parameter tissue compartment mapping," Magnetic Resonance in Medicine, vol. 79, p. 1950–1961, July 2017.
[6] W. D. Rooney and C. S. Springer, "A comprehensive approach to the analysis and interpretation of the resonances of spins 3/2 from living systems," NMR in Biomedicine, vol. 4, p. 209–226, October 1991.
[7] R. M. Kroeker and R. M. Henkelman, "Analysis of biological NMR relaxation data with continuous distributions of relaxation times," Journal of Magnetic Resonance (1969), vol. 69, p. 218–235, September 1986.
[8] O. Dietrich, J. G. Raya, S. B. Reeder, M. F. Reiser and S. O. Schoenberg, "Measurement of signal-to-noise ratios in MR images: Influence of multichannel coils, parallel imaging, and reconstruction filters," Journal of Magnetic Resonance Imaging, vol. 26, p. 375–385, 2007.
[9] P. B. Roemer, W. A. Edelstein, C. E. Hayes, S. P. Souza and O. M. Mueller, "The NMR phased array," Magnetic Resonance in Medicine, vol. 16, p. 192–225, November 1990.
[10] K. P. Pruessmann, M. Weiger, M. B. Scheidegger and P. Boesiger, "SENSE: Sensitivity encoding for fast MRI," Magnetic Resonance in Medicine, vol. 42, p. 952–962, November 1999.
[11] S. Aja-Fernández, G. V. Sánchez-Ferrero and A. Tristán-Vega, "Noise estimation in parallel MRI: GRAPPA and SENSE," Magnetic Resonance Imaging, vol. 32, p. 281–290, April 2014.
[12] D. C. Peters, J. A. Derbyshire and E. R. McVeigh, "Centering the projection reconstruction trajectory: Reducing gradient delay errors," Magnetic Resonance in Medicine, vol. 50, p. 1–6, June 2003.
[13] K. T. Block and M. Uecker, "Simple method for adaptive gradient-delay compensation in radial MRI," in Proceedings of the 19th Annual Meeting of ISMRM, Montreal, Canada, 2011.
[14] J. O. Cleary, S. Rot, M. Eyre, P. Bridgen, S. Dokumaci, Y. Blunck, W. Syeda, B. Solanky, S. Malik, M. Lim, C. Wheeler-Kingshott, S.-S. Tang and D. Carmichael, "Ultra-high field, multi-channel sodium MRI of the brain in children with epileptogenic SCN1A sodium channel mutations – a pilot study," in Submission to 2023 ISMRM & ISMRT Annual Meeting & Exhibition, 2022.
[15] A. H. Barnett, J. Magland and L. af Klinteberg, "A Parallel Nonuniform Fast Fourier Transform Library Based on an Exponential of Semicircle Kernel," SIAM Journal on Scientific Computing, vol. 41, p. C479–C504, January 2019.
[16] S. Lachner, L. Ruck, S. C. Niesporek, M. Utzschneider, J. Lott, B. Hensel, A. Dörfler, M. Uder and A. M. Nagel, "Comparison of optimized intensity correction methods for 23Na MRI of the human brain using a 32-channel phased array coil at 7 Tesla," Zeitschrift für Medizinische Physik, vol. 30, p. 104–115, May 2020.
[17] G. Madelin, J.-S. Lee, R. R. Regatte and A. Jerschow, "Sodium MRI: Methods and applications," Progress in Nuclear Magnetic Resonance Spectroscopy, vol. 79, pp. 14-47, 2014.
[18] B. Ridley, A. M. Nagel, M. Bydder, A. Maarouf, J.-P. Stellmann, S. Gherib, J. Verneuil, P. Viout, M. Guye, J.-P. Ranjeva and W. Zaaraoui, "Distribution of brain sodium long and short relaxation times and concentrations: a multi-echo ultra-high field 23Na MRI study," Scientific Reports, vol. 8, March 2018.
[19] S. Rosenzweig, H. C. M. Holme and M. Uecker, "Simple auto-calibrated gradient delay estimation from few spokes using Radial Intersections (RING)," Magnetic Resonance in Medicine, vol. 81, p. 1898–1906, November 2018.
[20] I. C. Atkinson, A. Lu and K. R. Thulborn, "Characterization and correction of system delays and eddy currents for MR imaging with ultrashort echo-time and time-varying gradients," Magnetic Resonance in Medicine, vol. 62, p. 532–537, April 2009.
[21] J. D. Ianni and W. A. Grissom, "Trajectory Auto-Corrected image reconstruction," Magnetic Resonance in Medicine, vol. 76, p. 757–768, September 2015.
[22] D. Burstein and C. S. Springer Jr, "Sodium MRI revisited," Magnetic Resonance in Medicine, vol. 82, pp. 521-524, 2019.
[23] W. Syeda, Y. Blunck, S. Kolbe, J. O. Cleary and L. A. Johnston, "A continuum of T2* components: Flexible fast fraction mapping in sodium MRI," Magnetic Resonance in Medicine, vol. 81, p. 3854–3864, January 2019.
[24] F. Riemer, B. S. Solanky, C. A. M. Wheeler-Kingshott and X. Golay, "Bi-exponential 23Na T2 component analysis in the human brain," NMR in Biomedicine, vol. 31, p. e3899, February 2018.
[25] T. A. Bjarnason, C. Laule, J. Bluman and P. Kozlowski, "Temporal phase correction of multiple echo T2 magnetic resonance images," Journal of Magnetic Resonance, vol. 231, p. 22–31, June 2013.
Figures