0019
Determining the minimum injected [2,3-2H2]fumarate concentration required for deuterium metabolic imaging of tumor cell death post-treatment1CRUK CI, University of Cambridge, Cambridge, United Kingdom, 2Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 3Department of Chemistry, University of Cambridge, Cambridge, United Kingdom, 4Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom
Synopsis
Keywords: Deuterium, Molecular Imaging
2H MRI measurements of labeled malate production following intravenous injection of 2H-labeled fumarate (1 g/kg) have been used to detect tumor cell death post-treatment in pre-clinical tumor models. We show here that much lower concentrations of 2H-labeled fumarate (0.1, 0.3 and 0.5 g/kg) can still generate a significant increase in malate concentration post-treatment, which should facilitate clinical translation. Mice were implanted subcutaneously with a triple-negative breast cancer model (MDA-MB-231) and treated with three different concentrations of a TRAIL-R2 agonist (MEDI3039). The different degrees of cell death obtained were linearly correlated with the malate/fumarate signal ratio and the absolute malate concentration.
Introduction
Imaging cell death can give an early indication of tumor treatment response and the effectiveness of therapy, where the degree of tumor cell death can be an indicator of treatment outcome. We have shown previously that deuterium MRI can be used to detect cell death and assess early tumor responses to treatment by measuring the increase in labelled malate concentration following intravenous [2,3-2H2]fumarate injection at a concentration of 1 g/kg (1,2). Fumarate is hydrated in the reaction catalyzed by the enzyme fumarase to produce malate. In necrotic cells, loss of plasma membrane integrity results in fumarate rapidly gaining access to the enzyme and an increased rate of malate production (1,3). Here, we investigated if lower concentrations of fumarate can be used to produce a similar increase in malate production in order to define a threshold [2,3-2H2]fumarate concentration for clinical translation. We also demonstrate that the concentration of malate produced is linearly correlated with the degree of cell death determined by histology.Methods
Mice, implanted subcutaneously with MDA-MB-231 cells, were injected with three different concentrations of [2,3-2H2]fumarate (0.1, 0.3 and 0.5 g/kg) before and after treatment with MEDI3039 (0.1, 0.4 and 0.8 mg/kg). The animals were anaesthetized and scanned using a 7T instrument (Agilent, Palo Alto, CA), as described previously (1,4). Thirteen serial 2H MR spectra were acquired over 65 min using a pulse-acquire sequence with a 2 ms BIR4 adiabatic excitation pulse (1,4), with a nominal flip angle of 67°, and a TR of 140 ms. Tumors were then excised and sections stained for a marker of cell death (TUNEL).Results
Deuterium-labeled fumarate, malate and water concentrations were assessed following intravenous injection of [2,3-2H2]fumarate (disodium salt) at 0.1, 0.3 and 0.5 g/kg (Figure 1). The malate/fumarate ratio increased significantly with increases in the concentration of injected [2,3-2H2]fumarate (Figure 2) at drug concentrations ≥ 0.4 mg/kg, where there were substantial increases in cell death (TUNEL positive cells) (Figure 3). Even at 0.1 g/kg fumarate there was a significant post-treatment increase in the malate/fumarate signal ratio at the higher drug concentrations (Figure 2 C,D). At the lower fumarate concentration (0.1 mg/kg) malate build-up was detectable at ~35 min post-injection (Figure 1S), whereas malate could already be detected by ~10 min following injection of higher (≥0.3 g/kg) fumarate concentrations (Figure 1 T,U). The increase in malate concentration was ~8-fold after fumarate injection at 0.3 g/kg and 0.5 g/kg and ~3-fold at 0.1 g/kg (Figure 3 B,D,F). Both the malate/fumarate ratio and malate concentration were linearly correlated with TUNEL staining of tumor cell death (P < 0.0002, R > 0.7) (Figure 3 A,C,E), however the relationship was weaker at 0.1 g/kg fumarate (malate/fumarate ratio vs TUNEL staining, slope=0.004; malate concentration vs TUNEL staining, slope=0.008) when compared to that at 0.3 and 0.5 g/kg injected fumarate concentration (malate/fumarate ratio, slope=0.089 and 0.031 respectively; malate concentration, slope=0.096 and 0.034 respectively).Discussion
[2,3-2H2]fumarate has considerable potential for the quantitative assessment of tumor cell death in vivo even at lower concentrations than that used previously (1g/kg). All three concentrations of [2,3-2H2]fumarate tested resulted in a substantial increase in malate production, with 0.3 and 0.5 g/kg generating malate concentrations > 1 mM, which should be sufficient for detection on clinical 3T MR scanners (2). The concentration of malate produced was linearly correlated with the degree of cell death and in this tumor model the production of 1 mM labelled malate equated to ~ 33% tumor cell necrosis.Conclusion
We showed previously that tumor cell death could be detected from the labelled malate produced following intravenous injection of 1 g/kg [2,3-2H2]fumarate. We have shown here that cell death can still be detected at 0.1 g/kg [2,3-2H2]fumarate but that 0.3 g/kg might be optimal for the detection of tumor cell death in the clinic.Acknowledgements
The authors acknowledge the support of the Cancer Research UK Cambridge Institute core facilities, in particular the biological resources unit, histopathology, and preclinical imaging sections. We would also like to thank Jodi Miller, Sarah McGuire, Dominick McIntyre and Madhu Basetti for their help. The work was supported by grants from Cancer Research UK (C197/A17242, C197/A16465, C9685/A25177). F.H. is in receipt of a Cambridge European Scholarship from the Cambridge Trust.References
1. Hesse F, Somai V, Kreis F, Bulat F, Wright AJ, Brindle KM. Monitoring tumor cell death in murine tumor models using deuterium magnetic resonance spectroscopy and spectroscopic imaging. Proc Natl Acad Sci U S A 2021;118: e20146311182.
2. Hesse F, Wright AJ, Somai V, Bulat F, Kreis F, Brindle KM. Imaging Glioblastoma Response to Radiotherapy Using 2H Magnetic Resonance Spectroscopy Measurements of Fumarate Metabolism. Cancer Res 2022;82:3622-333.
3. Gallagher FA, Kettunen MI, Hu D-E, Jensen PR, Zandt RIt, Karlsson M, et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc Natl Acad Sci U S A 2009;106:19801-64.
4. Kreis F, Wright AJ, Hesse F, Fala M, Hu DE, Brindle KM. Measuring Tumor Glycolytic Flux in Vivo by Using Fast Deuterium MRI. Radiology 2020;294:289-96
Figures
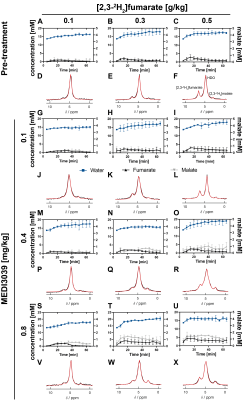
Figure 1: 2H MR spectroscopic measurements of labeled fumarate, malate and water concentrations in MDA-MB-231 tumors following injection of increasing concentrations (0.1 - 0.5 g/kg) of [2,3-2H2]fumarate (A-C, G-I, M-O, S-U) before (A-C) and 24 h after treatment with increasing concentrations of MEDI3039 (G-I (0.1 mg/kg, n=3), M-O(0.4 mg/kg, n=3), S-U (0.8 mg/kg, n=3)). The corresponding representative spectra, the sum of 12 spectra recorded over 60 min, are shown in (D-F), (J-L), (P-R) and (V-X).
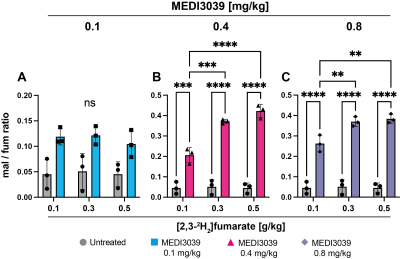
Figure 2: Malate/fumarate signal ratios in MDA-MB-231 tumors before (untreated n=3) and after MEDI3039 treatment at (A) 0.1 (n=3), (B) 0.4 (n=3, ***P < 0.001, ****P < 0.0001) and (C) 0.8 mg/kg (n=3, **P < 0.01, ****P < 0.0001). Ratios were obtained by summing the fumarate, malate and HDO signals over 60 min after injection of [2,3-2H2]fumarate at 0.1, 0.3 and 0.5 g/kg. Data are presented as mean ± SD. ns, not significant.
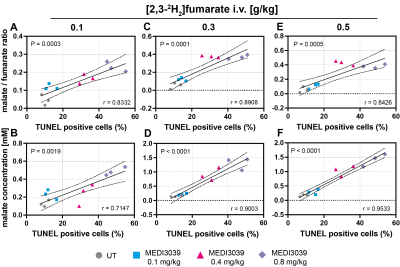
Figure 3: Pearson correlation analysis of a histological marker of cell death (TUNEL, % positive cells) with the malate/fumarate ratio and malate concentration (mM). MDA-MB-231 tumor-bearing mice, treated with MEDI3039 (0.1, 0.4 or 0.8 mg/kg, 24 h, i.v.). Two-tailed, Pearson p and r values are shown.