0018
Imaging choline phospholipid biosynthesis in gliomas using deuterium magnetic resonance spectroscopy1Radiology and Biomedical imaging, University of California San Francisco, San Francisco, CA, United States
Synopsis
Keywords: Deuterium, Cancer
Aberrant choline phospholipid biosynthesis is a metabolic hallmark of cancer. Choline kinase α (CKα) is the key enzyme in this pathway. Non-invasive methods of imaging CKα have the potential to report on tumor proliferation. Here, using a combination of RNA interference and doxycycline-inducible gene silencing systems, we show that deuterium magnetic resonance spectroscopy following administration of [2H9]-choline non-invasively reports on CKα activity in patient-derived glioma cells and intracranial tumors. Importantly, [2H9]-choline provides an early readout of response to chemotherapy in mice bearing intracranial gliomas. Our studies identify a novel agent for metabolic imaging of gliomas and their response to therapy.
Introduction
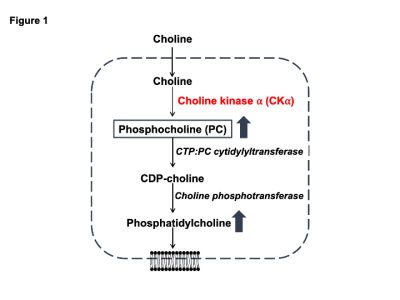
Elevated phospholipid biosynthesis is a hallmark of cancer1. Due to the high membrane turnover associated with uncontrolled proliferation, tumor cells upregulate phospholipid biosynthesis1. Choline is phosphorylated to phosphocholine (PC) and subsequently incorporated into phosphatidylcholine, which is the primary phospholipid in mammalian cells (Fig. 1). Choline kinase α (CKα) is the key enzyme in this pathway1. Identifying non-invasive methods of imaging CKα activity can enable assessment of tumor burden and response to therapy. Deuterium magnetic resonance spectroscopy (DMRS) following administration of 2H-labeled substrates is a clinically translatable method of imaging metabolic activity in vivo2. The goals of the current study were to determine whether [2H9]-choline tracks CKα activity and to establish the utility of [2H9]-choline for glioma imaging.Methods
DMRS in cells: Patient-derived glioblastoma (GBM1) and oligodendroglioma (BT88) cells were cultured as described previously3-6. CKα expression was silenced by RNA interference7,8. Non-targeting siRNA was used as control. Concomitantly, cells were incubated in media containing 56mM choline chloride-(trimethyl-d9) ([2H9]-choline) for 48h. 2H-MR spectra were acquired from live cells on a Varian 14.1T scanner using a 16mm 2H surface coil and a pulse-acquire sequence (TR=260ms, NA=2500, complex points=512, flip angle=64o, spectral width=2kHz)9. Peak integrals were normalized to the natural abundance of semi-heavy water (HDO, 4.75ppm) from a saline-containing vial9.In vivo CKα silencing: To determine whether [2H9]-choline tracks CKα in vivo, we engineered GBM6 cells to silence CKa in a doxycycline-inducible manner (henceforth referred to as GBM6doxCKα). GBM6doxCKα cells were intracranially implanted in SCID mice. Tumor volume was determined by T2-weighted MRI on a 14.1T scanner equipped with a 1H volume coil and a spin-echo multi-slice sequence10. Once tumors were ~30mm3, [2H9]-choline metabolism was evaluated. Following injection of a bolus of [2H9]-choline (200mg/kg) via a tail-vein catheter, non-localized 2H-MR spectra were acquired over 50min with a pulse-acquire sequence on a Bruker 3T scanner (TR=506.361ms, NA=1000, complex points=256, flip angle=64, spectral width=512.8, temporal resolution = 8min 26s). Mice were then treated with doxycycline (50mg/kg daily) and [2H9]-choline metabolism assessed. The signal to noise ratio (SNR) of PC was calculated. For spatial localization, 2D chemical shift imaging (CSI; TE/TR=1.04/265ms, FOV=30x30mm2, 8x8, 128 points, 2Hz spectral width, NA=30) was performed on a Bruker 3T scanner.
Response assessment: Patient-derived BT257 astrocytoma cells were intracranially implanted and tumor volume determined by T2-weighted MRI on a 14.1T scanner. Mice were treated with temozolomide (50mg/kg intraperitoneally). Non-localized DMRS was performed to evaluate [2H9]-choline metabolism before and after treatment on a 14.1T scanner (TR=500ms, NA=500, complex points=512, flip angle=64, spectral width=2kHz, temporal resolution = 4min 10s). Peak integrals were normalized to HDO.
Statistical analysis: Results were expressed as mean±standard deviation. Statistical significance was assessed using an unpaired two-tailed Welch’s t-test with p<0.05 considered significant.
Results
[2H9]-choline provides a readout of CKα activity: Previous studies indicate that a peak at 3.2 ppm can be observed using DMRS following administration of [2H9]-choline11,12. However, since choline, PC and glycerophosphocholine, a degradation product of phosphatidylcholine, all resonate at 3.2 ppm1,11,12, it is not clear whether this peak reflects choline metabolism to PC via CKα. To determine whether [2H9]-choline tracks CKα activity, we examined the effect of silencing CKα on [2H9]-choline metabolism in GBM1 and BT88 cells. We confirmed that CKα activity was significantly reduced in siCKα cells relative to siControl (Fig. 2A). Importantly, silencing CKα resulted in a complete loss of the 3.2 ppm peak following incubation with [2H9]-choline in both GBM1 and BT88 models, suggesting that this peak is PC (Fig. 2B-2C).To assess the validity of these results in an in vivo setting, we performed experiments with patient-derived GBM6doxCKα cells in which CKα was silenced in a doxycycline-inducible manner. We intracranially implanted mice with GBM6doxCKα cells and examined [2H9]choline metabolism before and after doxycycline treatment. As shown in Fig. 3A-3B, the peak at 3.2 ppm was completely lost upon CKα silencing in vivo, confirming that the 3.2 ppm peak represents PC. To further validate these results and assess the spatial distribution of [2H9]choline metabolism, we performed 2D CSI before and after doxycycline treatment in mice bearing intracranial GBM6doxCKα tumors. As shown in Fig. 4A, metabolic heatmaps showed localization of PC to the tumor vs. normal brain, an effect that was lost following CKα silencing. Collectively these results suggest that [2H9]choline provides a readout of CKα in vivo.
[2H9]-choline can be used to non-invasively monitor response to therapy: Next, we examined whether [2H9]-choline provides a readout of response to temozolomide (TMZ), which is a standard of care for glioma patients13. As shown in Fig. 5A, TMZ induced tumor shrinkage in BT257 tumor-bearing mice, an effect that was apparent by day 15. Importantly, PC production from [2H9]-choline was reduced in BT257 tumor-bearing mice at day 7 (Fig. 5B-5C), when no difference in tumor volume was detectable (Fig. 5A).
Conclusions
Elevated CKα activity is observed in most cancers, including gliomas. Non-invasive methods of assessing CKα activity are needed. Our studies indicate that [2H9]-choline provides a readout of CKα activity in patient-derived glioma cells and tumors. Importantly, [2H9]-choline provides an early readout of response to therapy, prior to MRI-detectable volumetric alterations. Clinical translation of [2H9]-choline has the potential to improve response assessment in glioma patients.Acknowledgements
This study was supported by NIH R01CA239288, Department of Defense W81XWH201055315 and the UCSF NICO initiative.
References
1 Glunde, K., Bhujwalla, Z. M. & Ronen, S. M. Choline metabolism in malignant transformation. Nature reviews. Cancer 11, 835-848, doi:10.1038/nrc3162 (2011).
2 De Feyter, H. M. & de Graaf, R. A. Deuterium metabolic imaging - Back to the future. Journal of magnetic resonance (San Diego, Calif. : 1997) 326, 106932, doi:10.1016/j.jmr.2021.106932 (2021).
3 Mancini, A. et al. Disruption of the beta1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer cell 34, 513-528 e518 (2018).
4 Giannini, C. et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncology 7, 164-176 (2005).
5 Kelly, J. J. et al. Oligodendroglioma cell lines containing t(1;19)(q10;p10). Neuro-oncology 12, 745-755, doi:10.1093/neuonc/noq031 (2010).
6 Mazor, T. et al. Clonal expansion and epigenetic reprogramming following deletion or amplification of mutant IDH1. Proceedings of the National Academy of Sciences of the United States of America 114, 10743-10748, doi:10.1073/pnas.1708914114 (2017).
7 Viswanath, P. et al. Mutant IDH1 gliomas downregulate phosphocholine and phosphoethanolamine synthesis in a 2-hydroxyglutarate-dependent manner. Cancer & metabolism 6, 3, doi:10.1186/s40170-018-0178-3 (2018).
8 Viswanath, P. et al. 2-hydroxyglutarate-mediated autophagy of the endoplasmic reticulum leads to an unusual downregulation of phospholipid biosynthesis in mutant IDH1 gliomas. Cancer research, doi:10.1158/0008-5472.can-17-2926 (2018).
9 Batsios, G. et al. Deuterium metabolic imaging reports on TERT expression and early response to therapy in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research, doi:10.1158/1078-0432.Ccr-21-4418 (2022).
10 Batsios, G. et al. PI3K/mTOR inhibition of IDH1 mutant glioma leads to reduced 2HG production that is associated with increased survival. Sci Rep 9, 10521, doi:10.1038/s41598-019-47021-x (2019).
11 De Feyter, H. T., Thomas, M., Ip, K., Behar, K. & de Graaf, R. Delayed mapping of 2H-labeled choline using Deuterium Metabolic Imaging (DMI) reveals active choline metabolism in rat glioblastoma. 2021 Annual Meeting of the International Society for Magnetic Resonance in Medicine (2021).
12 Veltien, A. et al. Simultaneous Recording of the Uptake and Conversion of Glucose and Choline in Tumors by Deuterium Metabolic Imaging. Cancers (Basel) 13, doi:10.3390/cancers13164034 (2021).
13 Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine 352, 987-996, doi:10.1056/NEJMoa043330 (2005).
Figures
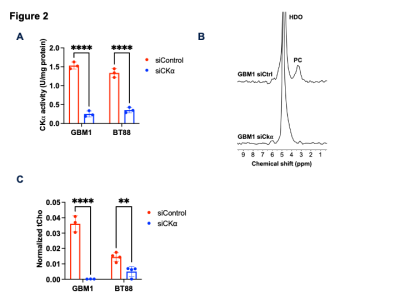
[2H9]-choline tracks CKα activity in glioma cells. (A) Verification of CKα silencing by quantification of CKα activity in siControl and siCKα cells for the GBM1 and BT88 models. (B) Representative 2H-MR spectra from GBM1 siControl and siCKα cells cultured in medium containing 56mM [2H9]-choline for 48h. Peaks for semi-heavy water (HDO; 4.75 ppm) and PC (3.2 ppm) are labeled. (C) Quantification of normalized PC in siControl and siCKα cells for the GBM1 and BT88 models. ** = p<0.01; **** = p<0.0001.
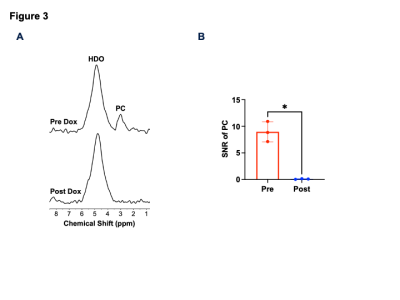
[2H9]choline provides a readout of CKα activity in vivo at 3T. (A) Representative summed 2H spectra acquired after intravenous injection of [2H9]-choline into a mouse bearing an orthotopic GBM6doxCKα tumor before (day 0; D0) and after (day 7; D7) doxycycline treatment. (B) Quantification of the SNR of PC in GBM6doxCKα tumor-bearing mice before and after doxycycline treatment. * = p<0.05.
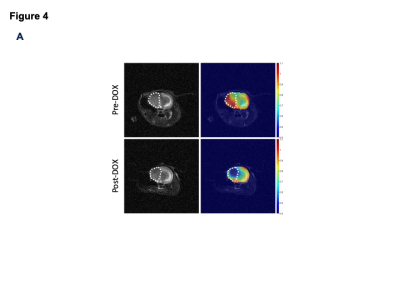
PC production is localized to the tumor region in vivo at 3T. Representative 2D CSI data from a mouse bearing an intracranial GBM6doxCKα tumor before (top row) and after (bottom row) CKα silencing by doxycycline. The left panel shows the T2-weighted MRI, and the right panel shows the heatmap of PC overlaid on the T2-weighted MRI. The tumor is contoured by white dotted lines.
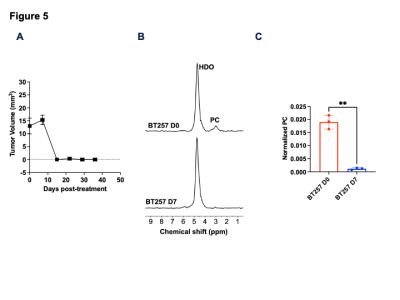
[2H9]-choline reports on early response to therapy in vivo. (A) Quantification of the effect of TMZ on tumor volume in mice bearing orthotopic BT257 tumors. (B) Representative summed 2H spectra acquired after intravenous injection of [2H9]-choline into a BT257 tumor-bearing mouse before (day 0; D0) and after (day 7; D7) TMZ. (C) Quantification of normalized PC in BT257 tumor-bearing mice at D0 and D7 of TMZ treatment (n=3).