0002
Fast Spin Echo Approach for Accelerated B1-gradient Based MRI1Center for Magnetic Resonance Research & Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research & Department of Radiology; Department of Biomedical Engineering, University of Minnesota, Minneapolis, MN, United States
Synopsis
Keywords: YIA, Gradients
Recent efforts to expand access to MRI have focused on low-cost, portable MRI systems that eliminate pulsed B0 gradients in favor of radio-frequency imaging techniques. In this work we present a new multi-echo version of FREE (Frequency-modulated Rabi Encoded Echoes) that utilizes nonlinear B1+ gradients to perform spatial encoding. This new technique leverages the acceleration of conventional FSE approaches and nonlinear gradients to eliminate the need for conventional B0 gradients while also achieving very high spatial resolution.
Introduction:
In this work, we present a multi-echo (ME) version of FREE (Frequency-modulated Rabi Encoded Echoes) based on the clinically proven fast spin echo (FSE) approach, where multiple echoes are collected during a single shot1. This new sequence leverages the acceleration of conventional FSE approaches but eliminates the need for conventional B0 gradients to perform phase-encoding2,3. In-vivo imaging produced comparable images to conventional FREE in a fraction of the time.Methods:
The new accelerated ME-FREE sequence expands upon the previous multi-shot FREE sequence by incorporating traditional FSE techniques, but replaces the conventional phase encoding gradients with new RF-based gradients. To achieve this acceleration, additional alternating adiabatic full passage (AFP) pulses are used to augment the phase of later echoes approximately linearly, thus traversing new points in k-space (Figure 1). The encoding scheme proposed for the new sequence employs a multi-shot, inwards-outwards sampling pattern to acquire both halves of k-space (Figure 2). To accomplish this, the frequency-sweep direction of the AFPs needs to be reversed, during both the echo train and the pair of initial pulses.Data were acquired with a CIERMag digital magnetic resonance spectrometer (DMRS)4-7, interfaced to a 1.5 T, 90-cm magnet with a clinical gradient. The magnet was initially designed to operate at 4 T but was ramped down to 1.5 T without adjusting the passive shims, leaving a relatively nonuniform B0. A single-loop surface coil was utilized to create the spatially-varying RF fields necessary for all in-vivo imaging experiments.
All ME-FREE sequences utilized the same HS48 pulses to acquire 4 echoes per shot, with 18 shots per initial FM sweep direction. A conventional double spin-echo (DSE) image was acquired using traditional phase encoding gradients (Figure 3). To study the different types of contrast possible with the ME-FREE sequence, three different TR values were used and an inversion pulse was added to create a FLAIR-type sequence (Figure 4)9.
Results:
The ME-FREE images utilized the spatially-varying B1+ of the surface coil to encode spatial information perpendicular to the coil; thus removing the conventional B0 gradient along that dimension. Distortions arising from the nonlinear B1+ gradient are illustrated when comparing the FFTs of the ME-FREE image to the traditional DSE image acquired using conventional B0 gradients (Figure 3A,C). However, undistorted FREE images can be produced with prior knowledge of the B1+ transmit field10 (Figure 3B). Additional images were acquired with three TR values (2.5, 4.5, and 5.5 s) while the pulse parameters were held constant between images (Figure 4A-C). As TR increased, the contrast shifted to a more T2 weighted image with the ventricles transitioning from dark to bright with each progressive image. With the addition of an inversion pulse to nullify CSF signal, ME-FREE demonstrated the ability to acquire a FLAIR image (Figure 4D). An analysis of the spatially-varying pixel resolution was performed utilizing the surface coil and the previous pulse parameters (Figure 5). Three traces through the resulting image highlight the variable resolution, with the characteristic horns corresponding to regions of very steep B1+ gradients with micron-level spatial resolution.Discussion:
The multi-echo FREE sequence presented in this work has demonstrated not only the capability of eliminating one pulsed B0 gradients but represents an important step in improving upon the original FREE technique. For example, the original multi-shot FREE had an average RF power delivered to the surface coil of ~3.6 W per 5-min to collect a 64 x 128 image (FREE × Frequency Encoding), whereas the new multi-echo sequence required 2.9 W per 5-min for a 125 x 128 image. This accomplished both a reduction in the averaged power delivered and image acquisition. As compared to the original implementation, the multi-echo sequence still achieved extremely fine spatial resolution with an acceleration factor of 3.9.Both implementations of the FREE method utilized a nonlinear B1+ field to perform the spatial encoding; thus, have a spatially variable resolution (i.e., Δk varies across the FOV). This leads to the unique distortions observed in the FFTs of the FREE images, with the regions close to the coil having a very steep B1+ field producing extremely fine resolution that is subsequently heavily sampled. By utilizing a reconstruction technique that employed a zero padding–type approach prior to distortion and sensitivity correction, the final undistorted image is scaled to its native high spatial resolution along the vertical dimension.
With the benefits of reduced total scan time and average power delivered, a more thorough analysis was performed on the types of contrast now possible. For example, by increasing TR at a fixed TE, the contrast gradually shifted to a more T2-weighted image with the ventricles transitioning from dark to bright. To further study the clinically relevant contrast, an inversion pulse was added before the initial excitation to create a FLAIR sequence. In this configuration, a FLAIR image only required 6 min to collect a 125×128 image.
Conclusion:
The multi-echo FREE technique introduced in this work shows the feasibility of eliminating a pulsed B0 gradient while accelerating image acquisition and maintaining high spatial resolution. In doing so, this work represents a significant step toward the development of low-cost MRI scanners that do not require expensive B0 gradient hardware and associated infrastructure.Acknowledgements
No acknowledgement found.References
1. Torres E, Froelich T, Wang P, et al. B-1-gradient-based MRI using frequency-modulated Rabi-encoded echoes. Magnet Reson Med. Feb 2022;87(2):674-685. doi:10.1002/mrm.29002
2. Hennig J, Nauerth A, Friedburg H. Rare Imaging - a Fast Imaging Method for Clinical Mr. Magnet Reson Med. Dec 1986;3(6):823-833. doi:DOI 10.1002/mrm.1910030602
3. Sharp JC, King SB. MRI Using Radiofrequency Magnetic Field Phase Gradients. Magnet Reson Med. Jan 2010;63(1):151-161. doi:10.1002/mrm.22188
4. Martins M, Vidoto E, Tannús A, inventors; Universidade de São Paulo, assignee. Espectrômetro para uso em sistemas de ressonância magnética e sistemas de ressonância magnética. Brazil patent BR102015000624-1. 2015.
5. Pizetta D, Lourenço G, Silva D, Vidoto E, Martins M, Tannús A. Magnetic resonance system configuration and editing tools. presented at: IUPESM 2015: Wold congress on medical physics & biomedical engineering; Jun 7-12 2015; Toronto, Canada.
6. Pizetta D, Shimada D, Falvo M, et al, inventors; PyMR - a framework for programming magnetic resonance systems. Brazil patent BR512019001829-0. 2017.
7. Tannús A, Pizetta D, Silva D, Vidoto E, Lourenço G, Martins M, inventors; Universidade de São Paulo, assignee. ToRM-IDE - Integrated development environment for magnetic resonance applications. Brazil patent BR512015001484-6. 2015.
8. Garwood M, DelaBarre L. The return of the frequency sweep: Designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153:155-177. doi:10.1006/jmre.2001.2340
9. Okuda T, Korogi Y, Ikushima I, et al. Use of fluid-attenuated inversion recovery (FLAIR) pulse sequences in perinatal hypoxic-ischaemic encephalopathy. Br J Radiol. Mar 1998;71(843):282-90. doi:10.1259/bjr.71.843.9616237
10. Wang P, Froelich T, Torres E, et al. Correcting image distortions from a nonlinear B1+ -gradient field in frequency-modulated Rabi-encoded echoes. Magn Reson Med. May 2023;89(5):2100-2108. doi:10.1002/mrm.29549
Figures
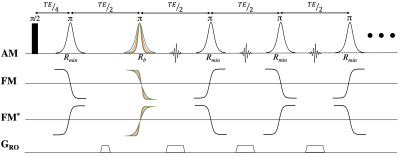
Figure 1: Multi-Echo FREE sequence utilizing a spatially varying B1+ and a frequency encoding gradient to acquire a 2D image. The phase difference created between the first pair of pulses controls the initial step size in k-space with the additional pulses adding a constant phase to step through k-space incrementally. FM and FM* control the direction of the first step in k-space, where alternating the FM sweep direction during the echo train flips the sign of the acquired k-space point.
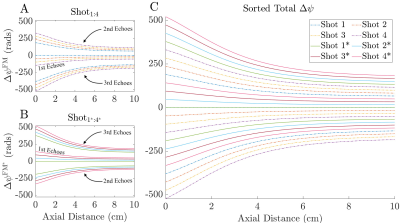
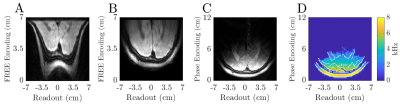
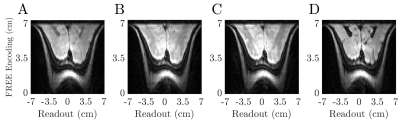
Figure 4: A demonstration of different contrasts with ME-FREE. All images have a matrix size of 125x128 (FREE x Frequency Encoding) and were acquired using the same pulse parameters as Figure 3. A) TR = 2.5 s and a total duration = 1.5 min. B) TR = 4.5 s and a total duration = 2.7 min. C) TR = 5.5 s and a total duration = 3.3 min. D) FLAIR image with a TR = 10 s and TI = 2.8 s and total duration of 6 min.
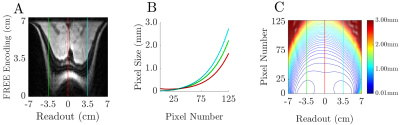
Figure 5: Analysis of the spatial variation of the pixel size. A) The three regions of interest correspond to the horns and along the central axis of the coil. B) The horns correspond to regions of very fine spatial resolution, dropping down to 10 μm in regions close to the coil. As the axial distance from the coil increases, the resolution degrades. C) A 2D contour plot showing the regions of constant resolution. At depth, the resolution continues to degrade as the transmit field approaches zero.