5063
Cardiovascular Magnetic Resonance (CMR) in the diagnosis of Sarcoidosis: A Case report1Cape Universities Body Imaging Centre, University of Cape Town, Cape Town, South Africa, 2Division of Human Biology, Department of Biomedical Engineering, University of Cape Town, Cape Town, South Africa, 3Department of Radiation Medicine, Groote Schuur Hospital, Cape Town, South Africa, 4Department of Medicine, faculty of Health Sciences, University of Cape Town, Cape Town, South Africa, 5The Hatter Institute, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa
Synopsis
Sarcoidosis is defined as a multisystem granulomatous disease of unknown origin, characterized by noncaseating granulomas. In cardiac sarcoidosis (CS), granulomas have been linked to ventricular tachyarrhythmias and electrical instability. Cardiovascular magnetic resonance (CMR) can provide diagnostic information and aid the clinical management. Here, we report a case of a middle-aged male patient with intermittent complete heart block and with a history of hypertension and diabetes mellitus. He was referred for evaluation of CS. Subsequently, the patient underwent successful cardiac pacemaker implant and remains clinically well.
Background
Cardiac sarcoidosis (CS) occurs in about 50% of patients with systemic sarcoidosis and only an estimated 5% patients have clinical manifestations, making diagnosis difficult 1, 2, 3. CMR has been successfully employed to accurately assess left ventricular size and function, identify the distribution of the scar patterns, and allow for tissue characterization 4. We report on a 44-year-old male patient who presented with intermittent complete heart block to the emergency department. He reported progressive shortness of breath over one-month, productive cough and swelling of the lower limbs. His co-morbidities include smoking, Type 2 diabetes, and poorly controlled hypertension. Chest x-ray showed multiple interstitial infiltrates. Echocardiogram revealed a restrictive filling pattern of the left ventricle (LV) with focal areas of increased density in the myocardium. CMR was requested for suspected CS.Cardiovascular magnetic resonance
The patient was scanned on a 3 Tesla, Siemens, Magnetom Skyra scanner, using an eighteen-channel body-array coil and integrated 32-channel spine coil, in a supine position. Scanning protocol included: cine imaging, cine tagging, T1 and T2 maps, and late gadolinium enhancement imaging 3. The CMR protocol and parameters of the subsequent sequences are summarizes in Table 1.Findings
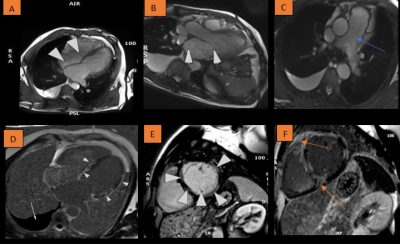
Severe systolic dysfunction, with an ejection fraction of 25% and biventricular dilation was confirmed. The left atrium (LA) size was severely enlarged and there was severe mitral and tricuspid regurgitation. Right regional wall motion abnormalities present included mid to apical right ventricular (RV) dyskinesia and aneurysms. Moreover, multiple patterns were seen on LGE suggest a likely diagnosis of CS with biventricular involvement (as seen in fig.1). Additionally, the main pulmonary artery (PA) was enlarged, with moderate right pleural effusion and lung infiltration.Teaching points
- CS can lead to multiple clinical manifestations include LV dysfunction, arrhythmia and sudden cardiac death 4, 6, 7.
- Cardiac involvement in sarcoidosis can be robustly and reproducibly detected using CMR and assist in clinical management.
- Patchy, often bright LGE in a non-coronary distribution.
- LGE may represent a combination of active granuloma infiltration and fibrosis § Granulomas and inflammation are present during acute phase.
- T1-weighted sequences are used for assessing inflammation, fibrosis, and infiltration.
- T2-weighted short tau inversion recovery (STIR) sequence can be used to suppress signals from specific tissues, moving blood and fat, making it useful in the detection of oedema 8.
Conclusion
CMR is a powerful tool for diagnosis, risk stratification, and evaluating efficacy of therapeutic intervention. LGE is an independent predictor of adverse outcome in sarcoidosis. Early identification of cardiac sarcoidosis has been shown to improve treatment planning and preventing subsequent progression to arrhythmia and heart failure 5.Acknowledgements
I want to thank Zanele Mlilo and Morne Kahts for helping with CMR images.References
1. Sharma A, Okada DR, Yacoub H, Chrispin J, Bokhari S. Diagnosis of cardiac sarcoidosis: an era of paradigm shift. Ann Nucl Med [Internet]. 2020;34(2):87–93.
2. Shade JK, Prakosa A, Popescu DM, Yu R, Okada DR, Chrispin J, et al. Predicting risk of sudden cardiac death in patients with cardiac sarcoidosis using multimodality imaging and personalized heart modeling in a multivariable classifier. Sci Adv. 2021;7(31).
3. Juneau D, Nery PB, Pena E, Inácio JR, Beanlands RSB, deKemp RA, et al. Reproducibility of cardiac magnetic resonance imaging in patients referred for the assessment of cardiac sarcoidosis; implications for clinical practice. Int J Cardiovasc Imaging [Internet]. 2020.
4. Kagioka Y, Yasuda M, Okune M, Kakehi K, Kawamura T, Kobuke K, et al. Right ventricular involvement is an important prognostic factor and risk stratification tool in suspected cardiac sarcoidosis: analysis by cardiac magnetic resonance imaging. Clin Res Cardiol [Internet]. 2020;109(8):988–98.
5. Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging [Internet]. 2013;6(4):501–11.
6. Von Knobelsdorff-Brenkenhoff F, Schulz-Menger J. Role of cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology. J Cardiovasc Magn Reson [Internet]. 2016;18(1):1–18. 7. Orii M, Tanimoto T, Ota S, Takagi H, Tanaka R, Fujiwara J, et al. Diagnostic accuracy of cardiac magnetic resonance imaging for cardiac sarcoidosis in complete heart block patients implanted with magnetic resonance-conditional pacemaker. J Cardiol. 2020;76(2):191–7.
8. Steckman DA, Schneider PM, Schuller JL, Aleong RG, Nguyen DT, Sinagra G, et al. Utility of cardiac magnetic resonance imaging to differentiate cardiac sarcoidosis from arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol [Internet]. 2012;110(4):575–9.
Figures