5076
Whole Body DWI at 3T with Inline Geometric Distortion Correction and Broadband RF Inversion1Mermaid Beach Radiology, Gold Coast, Australia, 2Philips Healthcare ANZ Clinical Science, Sydney, Australia
Synopsis
The purpose of this work is to reverse geometric distortion uniformly across all stations of whole body Diffusion Weighted Imaging (DWI) at 3T for near perfect co-registration with cartesian imaging and correct alignment between stations for improved diagnostic confidence. This translation of a well established and published research technique translated in to clinical in-line application for geometric distortion correction in Whole Body DWI applications at 3T has revolutionised our confidence further in challenging regions of the body such as the thorax as well as vastly improved geometric stitching of multiple stations.
Purpose
The purpose of this work is to reverse geometric distortion uniformly across all stations of whole body Diffusion Weighted Imaging (DWI) at 3T for near perfect co-registration with cartesian imaging and correct alignment between stations for improved diagnostic confidence. Whole body DWI is often used in conjunction with PET scanning for detection of cancer for whole body screening, staging and surveillance during therapy such as Multiple myeloma and Lymphoma(1). The base sequence used, Single Shot Echo Planar (SS-EPI) based DWI, has been a clinical tool in MRI for the detection of stroke and other pathologies. This sequence relies on a homogenous field and optimised parameters for its diagnostic effectiveness as EPI sequences are intrinsically prone to local susceptibility, geometric distortion, Nyquist ghosting and fat suppression challenges. Initially designed for brain imaging which has a relatively spherical shape, regions outside of the brain are more challenging due to the changes in shape and larger air tissue interfaces which further challenge the SS-EPI sequence design, especially at 3T. STIR based fat suppression is used for robustness in these regions. There have been well established and published methods for geometric distortion correction in the brain (3,4) and this work translates this technique into whole body DWI clinical applications.Method
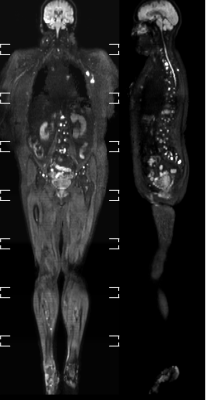
25 whole body scans using multi station Axial DWI (geometrically corrected), Cor STIR and Axial 3D T1 Dixon were evaluated for quality of co-registration with 3D t1 Dixon data. Scans were performed on a Philips Elition X 3T (Philips Netherlands). The Axial DWI sequences used a well established reverse gradient for the correction of geometric distortion (3,4). A b0 image with no diffusion gradients with the water-fat phase direction in the anterior-posterior and then posterior-anterior directions to create opposite directions of geometric distortion were processed establishing a b0 map to correct the geometric distortion. This has recently become an in-line process in current clinical scanners for DWI and Diffusion Tensor Imaging (DTI) applications in brain. This process was programmed to occur in-line for highest efficiency. The background b0 map parameters were optimised for the field of view and coverage per station. The DWI b values measured were b0 and b1000. and the voxel size was acquired at 4.5 x 4.5 x 5mm with no gap and interpolated to 2.25 x 2.25 x 5mm. All DWI stations were performed with free breathing with an overlap of 50mm. Customisation of broadband inversion radio-frequency pulses was introduced as well as optimised opposing readout polarisation using even number of data samples for optimised fat and background suppression.Results
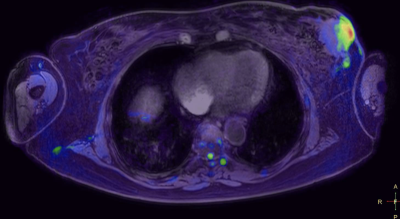
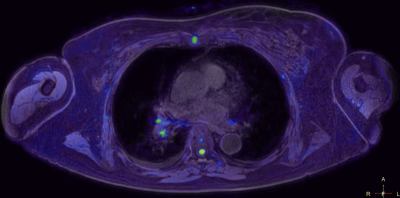
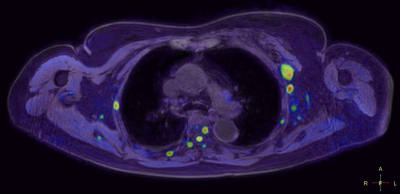
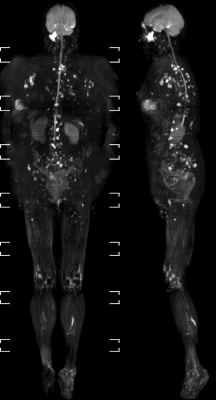
The co-registration of data with the 3D cartesian based T1 demonstrated very high accuracy in all stations for all patients. Pathology in regions such as lung and mediastinum accurately identified pathological changes which could be validated anatomically in both whole body STIR and T1 imaging (Figure 1a/b/c). There was a very consistent high accuracy in stitching between stations. This made it possible for seamless stitching of axially rotating maximum intensity projections and multi-planar reconstruction in both coronal and sagittal planes (Figures 2,3). This has been historically difficult for spine anatomy without this geometric correction. The modified broadband RF inversion pulse and opposed polarity readout for even data sets produced a high and uniform signal to noise ratio throughout each station as well as high consistency between stations. In 3 cases, heavy breathing produced a subtle ghosting artefact from the anterior chest motion in to the which may be due to respiratory differences between measurements however this had no impact on diagnostic significance.Discussion
This translation of a well established and published research technique translated in to clinical in-line application for geometric distortion correction in Whole Body DWI applications at 3T has revolutionised our confidence further in challenging regions of the body such as the thorax as well as vastly improved geometric stitching of multiple stations. Whilst whole body DWI scans are currently used in conjunction with PET-CT, a typical CT-PET dose has been found to be higher than most other diagnostic radiology exams and can reach over 35mSV so all efforts should be made to justify its benefit vs risk (5,6,7). This non ionising MRI aids in safer real time medicine where drugs and treatments can change based on regular serial scans to supplement PET CT scans and can be used to validate each other’s findings. Using in line reverse phase correction has previously demonstrated superior for the detection of cancer in pulmonary nodes when compared to PET-CT (2). Direct coronal acquired applications of this technology have seen the same results for dedicated sites such as abdomen and thorax in our clinical work. These improvements, used in conjunction with STIR and T1 sequences, will further increase diagnostic sensitivity and specificity in the assessment of cancer in comparison to what is already considered acceptable standards in current supplementary clinical use. More fine tuning developments will continue this exciting application for future commercial/clinical application availability.Acknowledgements
Mermaid Beach Radiology
Philips Healthcare ANZ Clinical Sciences Collaboration
Dr Iain Ball
Dr Zane Sherif
References
1. Albano et al, Whole Body Magnetic Resonance Imaging: Current Role in Patients with Lymphoma, Diagnostics2021, 11, 1007. https//doi.org/10.3390/diagnostics110610072.
2. Colletti, Reverse Phase Encoding-Corrected DWI Improves MRI or PET/MRI Lung Cancer, Radiology, Vol 295, No 3, https//doi.org/10.1148/radiol.20202001553.
3. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging, Holland et al, Neuroimage, 2010, 50(1):175 Elsevier4.
4. Correction of spatial distortion in EPI due to inhomogeneous static magnetic fields using reverse gradient method, Morgan et al, J Magn Reson Imaging, 2004; 19(4):499-507, foi:10.1002/jmri.200325.
5. Huang et al, Whole Body PET/CT Scanning: Estimation of Radiation Dose and Cancer Risk, Radiology, Vol 251, No1, https//doi.org/10.1148/radiol.25110813006.
6. International Atomic Energy Agency/ Home/ Resources/ Radiation Protection of Patients/ Health professionals/ Nuclear Medicine/ PET/CT, https://wwwiaea
7. Akin et al, Optimizing Oncologic FDG-PET/CT Scans to Decrease Radiation Exposure, https://www.imagewiseley.org/Imaging-Modalities/Nuclear-Medicine/Optimizing-Oncologic-FDG-PETCT-Scans
Figures