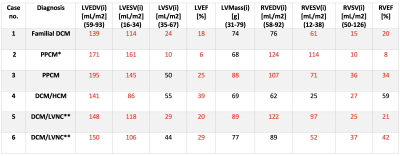
5061
Role of cardiovascular magnetic resonance in differentiating peripartum cardiomyopathy from pregnancy unmasking of underlying cardiomyopathy1Cape Universities Body Imaging Centre, University of Cape Town, Cape Town, South Africa, 2Division of Human Biology, Department of Biomedical Engineering, University Of Cape Town, Cape Town, South Africa, 3Cape Heart Institute, Faculty of Health Sciences,, University of Cape Town, Cape Town, South Africa, 4Division of Cardiology, Department of Medicine, University of Cape Town, Cape Town, South Africa, 5Department of Radiation Medicine, Groote Schuur Hospital, University of Cape Town, Cape Town, South Africa
Synopsis
Peripartum cardiomyopathy (PPCM) is a form of heart failure associated with pregnancy.1,2 We evaluated cardiovascular magnetic resonance (CMR) scans on 6 patients with PPCM and incomplete recovery. CMR showed an increase in left ventricular volumes and impairment of left ventricular ejection fraction (6-39%) in all patients. Right ventricular dysfunction was present in 5 patients. Linear mid-wall late gadolinium enhancement was present in all cases. Two patients had PPCM, one had dilated cardiomyopathy (DCM) with hypertrophy, 2 had DCM with left ventricular non-compaction [>6 months postpartum], and one had familial DCM. CMR is useful in distinguishing PPCM from other DCM phenotypes.
Background
Peripartum cardiomyopathy (PPCM) is a form of pregnancy-associated heart failure, affecting all ethnic groups worldwide. It may be a life-threatening disease, and considered a diagnosis of exclusion.1,2,3,5 Although the aetiology of PPCM remains unclear, risk factors include the following: females > 30 years of age, African descent, multiple parity, and multiple gravidity. PPCM has a number of clinical similarities to dilated cardiomyopathy (DCM) such as left ventricular (LV) dilatation, impaired systolic function and arrhythmias. Although the LV may not always be dilated in PPCM, LV ejection fraction (LVEF) is typically <45%. While the natural history of PPCM may range from rapid deterioration to full recovery, overall outcomes appear better in patients with PPCM in comparison to DCM, where full recovery of LV function is less common.3 There is a paucity of studies using CMR to evaluate cardiomyopathy phenotypes presenting in the peripartum period.4 Therefore, we sought to evaluate the differences between PPCM and DCM on CMR.Objectives
1. Study the CMR phenotypes of patients presenting with peripartum heart failure due to cardiomyopathy with incomplete recovery of their LV function. 2. Determine if there are CMR features that distinguish PPCM from other DCM phenotypes.Methods
We evaluated the CMR scans of 6 female patients with a preliminary diagnosis of PPCM who presented with heart failure during the postpartum period, with incomplete recovery of their LV function at follow-up (≥ 6 months). CMR studies were performed ≥ 6 months post-delivery in all cases, on both 3T, Siemens, Magnetom Skyra scanner and 1.5T Siemens, Magnetom Aera scanners. Imaging protocol included cine imaging, native T1 mapping, T2 mapping, late gadolinium enhancement (LGE) and post gadolinium T1 mapping.Results
Clinical characteristics: The mean age of presentation was 29.5 ±7.5 years. All patients had onset of symptoms postpartum. All patients had significantly impaired effort tolerance (New York Heart Association, NYHA Class III, n=1; NYHA Class IV, n=5) and symptoms of heart failure at baseline. One patient (table 1: case 5) had co-existing HIV on antiretroviral therapy. None of the patients reported a family history of cardiomyopathy or sudden cardiac death, but one patient reported a family history of heart failure (table 1: case 1). CMR characteristics. All parameters for LV and right ventricular (RV) function were indexed to body surface area (BSA). We observed a marked increase in indexed LV end-diastolic volume (LVEDVi) and LV end-systolic volume (LVESVi) in all 6 patients (Table 1). A combination of severe LV and RV dysfunction was observed in 5/6 patients, with LVEF ranging between 6-29% and RVEF 8-42%, respectively. LGE was present in all 6 patients; linear circumferential mid-wall LGE in 4 patients, septal linear mid-wall LGE in 2 patients . Annular dilatation resulted in moderate-to-severe functional mitral regurgitation in 5 patients, and mild and moderate-to-severe tricuspid regurgitation in 1 and 3 patients, respectively. Additional findings included a RV thrombus with a lung wedge infarct (table 1: case 3), multiple small liver cysts (table 1: case 4) and left upper lobe opacities in keeping with tuberculosis (table 1: case 5). Two patients had a final diagnosis of PPCM (where no other cause for DCM was identified) and 4 patients had evidence of an underlying intrinsic cardiomyopathy; one patient had a DCM with septal hypertrophy, 2 patients had DCM with left ventricular non-compaction (LVNC) overlap, and one patient had familial DCM with a confirmed pathogenic genetic mutation. While the presence of LGE or functional and volumetric analysis was not useful in distinguishing PPCM from other DCM phenotypes in this series, the presence of non-compaction and hypertrophy found on CMR was diagnostically useful.Teaching points
1. PPCM is a diagnosis of exclusion and it is important to consider alternative causes, particularly in patients that do not show improvement in LV size and function at follow-up. 2. In this series of cases, CMR was useful to distinguish overlapping phenotypes, namely DCM/LVNC. As normal remodelling in pregnancy can mimic LVNC, the diagnosis of LVNC can only be confirmed ≥ 6 months post-delivery. 3. CMR may be useful in identifying intracardiac thrombus or extracardiac features that may be clinically important, such as anticoagulation or treatment of non-cardiac pathologies as shown in this series. 4. Septal LGE may be present in both PPCM and other DCM phenotypes, and has been shown to be associated with poorer outcomes (in other studies). The presence of LGE may be helpful to identify individuals with PPCM at higher risk of adverse outcomes. 5. RV dysfunction is associated with poorer outcomes in patients with heart failure, and may be useful in predicting poorer outcomes in PPCM if present. The PPCM patient that died had severe biventricular dysfunction.Conclusion
PPCM and DCM are diseases that require prompt diagnosis and treatment to improve outcomes. It is important to consider the differential diagnosis in patients that fail to recover their LV function. CMR is an important diagnostic tool to aid in the diagnosis, risk stratification and clinical management of patients presenting with cardiomyopathy in the peripartum period.Keywords
Cardiovascular magnetic resonance imaging, peripartum cardiomyopathy, dilated cardiomyopathy, late gadolinium enhancementAcknowledgements
Mazwi Maishi
Solomon Aremu
Morne Kahts
References
1. Haghikia A., Röntgen P. & Vogel-Claussen J. et al. Characterization of peripartum cardiomyopathy by cardiovascular magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance. 2015. 17(Q46).
2. Sliwa K., Petrie MC, Hilfiker-Kleiner D, Mebazaa A, Jackson A, Johnson MR, van der Meer P, Mbakwem A. & Bauersachs J. Long-term prognosis, subsequent pregnancy, contraception and overall management of peripartum cardiomyopathy: practical guidance paper from the Heart Failure Association of the European Society of Cardiology Study Group on Peripartum Cardiomyopathy. European Journal of Heart Failure. 2018. 20(6):951-962.
3. Sliwa K., Bauersachs J., Arany Z., Spracklen TF & Hilfiker-Kleiner D. Peripartum cardiomyopathy: from genetics to management. European Heart Journal. 2021. 42(32): 3094–3102.
4. Petryka-Mazurkiewicz J., Kryczka K., Mazurkiewicz L., Miłosz-Wieczorek B., Spiewak M., Marczak M., Henzel J., Grzybowski, J., Demkow M. & Dzieli ´nska Z. Cardiovascular Magnetic Resonance in Peripartum Cardiomyopathy: Comparison with Idiopathic Dilated Cardiomyopathy. Diagnostics. 2021. 11: 1752.
5. Liang YD., Xu YW. & Li WH. et al. Left ventricular function recovery in peripartum cardiomyopathy: a cardiovascular magnetic resonance study by myocardial T1 and T2 mapping. Jornal of Cardiovascular Magnetic Resonance. 2020. 22(2).
Figures
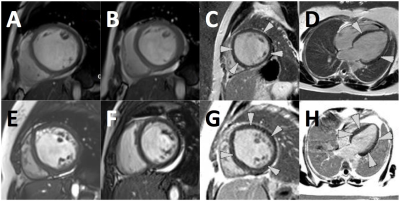
A. Short axis (SA) cine (end-diastole) in patient with PPCM.
B. SA cine (end-systole) in PPCM.
C. SA LGE view demonstrating linear pattern of enhancement (arrows).
D. 4-Chamber PSIR showing linear enhancement in septum and lateral wall.
E. SA cine (end-diastole) in patient with DCM showing hyper trabeculations in LV and RV.
F. SA cine (end-systole) showing pericardial effusion.
G. SA PSIR view showing linear circumferential enhancement (arrows).
H. 4-chamber PSIR showing LGE in septum and lateral wall of the LV.