5072
Non-Invasive MRI of Putamen Using Fused T1 MPRAGE and IR TSE FLAIR
Anna M Borkon1, Michael J DiBalsi2, Amy Muehlmatt3, Geary M Smith3, and Timothy H Lucas4
1MRI Research, University of Pennsylvania, Philadelphia, PA, United States, 2MRI Interventions, ClearPoint, Irvine, CA, United States, 3Comparative Medicine Services Core, CHOP Research Institute, Philadelphia, PA, United States, 4Neurosurgery, University of Pennsylvania, Philadelphia, PA, United States
1MRI Research, University of Pennsylvania, Philadelphia, PA, United States, 2MRI Interventions, ClearPoint, Irvine, CA, United States, 3Comparative Medicine Services Core, CHOP Research Institute, Philadelphia, PA, United States, 4Neurosurgery, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
The innovative method of fusing T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) and inversion recovery turbo spin echo fluid-attenuated inversion recovery (IR TSE FLAIR) provides the whole brain image with improved tissue contrast and distinguishes the boundaries within the subcortical structures of the basal ganglia. The presented experiment uses non-invasive MRI-guided imaging to obtain an adequate therapeutic coverage infusing the AAV test article to the right and left Putamen. Results have shown that fused MPRAGE and IR TSE FLAIR acquisitions obtained non-invasively provides more reliable and precise imaging of the right and left Putamen targeted for gene therapy.
Summary of Main Findings
The novel method of fusing MPRAGE and IR TSE FLAIR sequences provides the image with better coverage and tissue contrast and distinguishes boundaries of right and left Putamen within subcortical structures of the basal ganglia.Body of the Abstract
BACKGROUND MRI anatomical brain images used commonly for gene therapy as a pre-operative tool are based on standard contrast that doesn’t clearly distinguish the boundaries of the Putamen within subcortical nuclei of the basal ganglia. (Fig.1.).This poor image of target region can lead to inaccurate infusate coverage and therapeutic inefficacy. In the search for improvement of the guidance for the subcortical nuclei, mainly the Putamen, we used Cynomolgus Macaques models to test the innovative method of fusing T1-weighted sequence with 3-Dimentional magnetization prepared rapid acquisition gradient echo (MPRAGE) and inversion recovery turbo spin echo fluid-attenuated inversion recovery (IR TSE FLAIR). The fused image provided the whole brain with greater tissue contrast allowing for improved images of the right and left Putamen with distinguished borders within subcortical structures in the basal ganglia. MATERIALS AND METHODS 1. Non-Human Primate Subjects The experiment used 15 male Cynomolgus Macaques weighing 5.0–10.0 kilograms, 4-6 years of age. Each of them were sedated with dexamethasone (2 mg/kg) and telazol (3-5 mg/kg) with endotracheal intubation, followed by lubrication of the eyes, head shaving, intravenous site injection, physiological monitoring, and CSF and blood collection. 2. AAV Infusion AAV dose of 50μl was delivered to the right Putamen followed by a dose of 40μl to left Putamen by Medifusion pump starting at 1 μl/min (for 5 minutes), then at 2 μl/min (for 5 minutes), and ending at 3 μl/min (CHOP 2020). 3. Surgical Procedure MRI-guidance directed the surgeon with location of target, surgery planning, tower attachment, navigation to target, cannula trajectory and monitoring. Once the primate’s head was placed into 4-point cranial Smart Frame in the supine position and fixed to the right and left skull hemispheres with Siemens 4-channel coil, the monkey was moved into isocenter of 3T Siemens Magnet. 3.1. MRI Imaging Protocol Protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania. Initially, a 3-plane, 27-second scan was acquired to confirm the head position in the scanner. Then, cefazolin (20 mg/kg) was administered and head prepped with Chlorhexidine. Next, a sterile field was established and a grid was attached to the skull for planning the cannula trajectories. Then, a T1-weighted MPRAGE scan was acquired with MR-visible fiducial markers to confirm precise target location. MPRAGE acquisition with inversion recovery time (TI) of 900 ms, 9-degree flip angle (FA), bandwidth of 240 Hz/Px, echo time (TE) of 3.43 ms, repetition time (TR) of 2,300 ms, and 256-mm field of view (FOV) with 176 slices per slab used 180 degree inversion pulse followed by gradient echo. The 4:40-minute scan with isotropic voxels of 1.0×1.0×1.2 mm provided the whole brain with high resolution and tissue contrast. Next, IR TSE FLAIR was scanned with inversion time (TI) of 200 ms to null the signal intensity from cerebrospinal fluid (CSF). This was followed by a 180 degree radio frequency (RF) pulse, voxels of 1.1x1.0x2.0 mm over a 260-mm field of view (FOV), 27 slices of 2 mm of thickness, echo time (TE) of 44.0 ms, repetition time (RT) of 3,000 ms, 150-degree flip angle (FA) and bandwidth of 208 Hz/Px. The whole brain scan with greater tissue contrast was obtained in 7:56-minutes. (Fig.2.). Next, MPRAGE and IR TSE FLAIR were evaluated for MRI quality based on key metrics, comparing Signal-to-Noise Ratio (SNR) of the tissue scanned and Contrast-to-Noise Ratio (CNR) between the two adjacent tissues. An optional SNR of 1:00 was established for both acquisitions. The signal strength resulted in a good CNR and effective contrast. (Fig.3). Next, MPRAGE and IR TSE FLAIR were fused to precisely establish the boundaries of the target and planning trajectories. The tower was mounted to the scull to adjust the trajectory location by the ‘pitch and roll’ and X-Y axes. Next, the 2-D Fast Gradient Echo axial alignment scan was obtained in 13-seconds to detect position of cannula. For cannula tip placement to the target trajectory, a 3-D Fast Gradient Echo (Ortho 1) and (Ortho 2) were used until satisfactory minimal error confirmation was achieved. (Fig.4.). Then, the hole in the scalp was drilled. Next, the cannula was advanced to the right Putamen with proper depth and the infusion started. Pre-infusion MPRAGE was acquired and used for placement reassessment. Once finalized, post-MPRAGE was then applied to confirm the dispersion of AAV. The same process was reapplied for infusion into the left Putamen. The accuracy of AAV was confirmed on MPRAGE image. 3.2. Procedure Completion The catheter was withdrawn, the skull incision was closed with suture, and the monkey removed from magnet.Acknowledgements
Research work was sponsored by private Bio company.References
CHOP, Scientists. "Pre-Clinical Pharmacology/ AAV Putamen Dosing in NHPs." Protocol, Philadelphia, PA, 2020.Figures
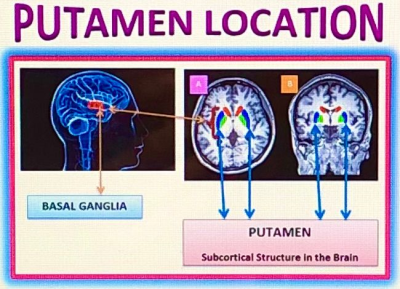
Fig.1.
The Putamen, the subcortical structure of the basal ganglia, is similarly
located in NHP’s brain; dysfunction of the Putamen can cause a number of
neurodegenerative disorders, but mainly it’s linked to problems with planning
and executing normal motor functions, as in Huntington’s Disease (HD) and
Parkinson’s Disease (PD); MRI human brain images with subcortical structures
(A) axial plane and (B) coronal plane
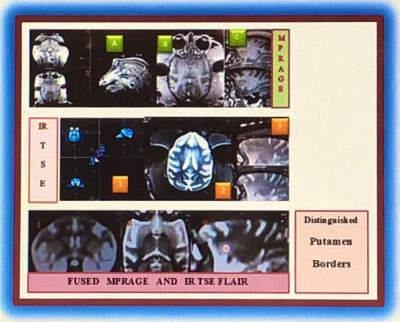
Fig.2.
MRI images of NHP brain with subcortical structures: T1-weighted MPRAGE (A)
3-Plane image, (B) Transversal Plane, (C) Sagittal Plane; IR TSE FLAIR (1)
3-Plane image, (2) Transversal Plane, (3) Sagittal Plane; Fused MPRAGE and IR
TSE FLAIR images with distinguished Putamen borders (I) Coronal Plane, (II)
Transversal Plane, (III) Sagittal Plane with marked Putamen location
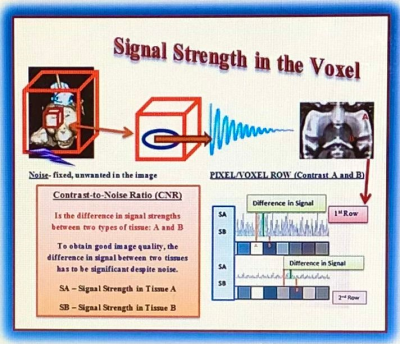
Fig.3.
Evaluation of MR image by the different pixel gray scales. MR raw data was computed using Fourier Transformation, thus each voxel on the image was
assigned signal intensity responding with specific grey value: Shown is a
single raw image of 8 pixels/voxels to differentiate the contrast in the image
between tissue A and B. Bright voxels result in a strong signal and dark voxels
result in a weaker signal. As a result, the 1st row image has lower CNR
and effective contrast caused by high noise in relationship to the contrast; 2nd row image has good CNR and effective contrast.
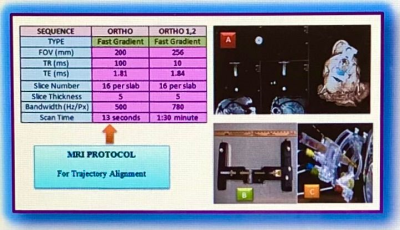
Fig.4.
NHP Brain Protocol parameters for Fast Gradient Sequences (Ortho and Ortho 1,
2) were used to adjust trajectory alignment for cannula insertion into the right
and left Putamen; (A) Orthogonal 3-Plane image with 3-D head rotation, (B)
Smart Frame for the NHP brain attachment in supine position, (C) Tower allowing
the surgeon to adjust the desired trajectory location in 4-planes using ‘pitch
and roll’ and X-Y axes
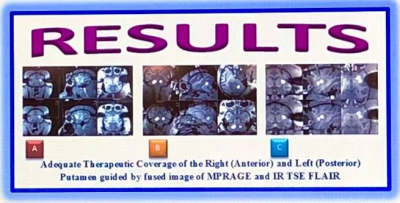
Fig.5.
MRI images of NHP brain with AAV article was precisely delivered to the Putamen
using fused image of MPRAGE and IR TSE FLAIR; (A) 3-Plane MPRAGE image of therapeutic
coverage to the right Putamen, (B) 3-Plane MPRAGE image of therapeutic coverage
to the left Putamen, (C) 3-Plane MPRAGE image of therapeutic coverage to the right
and left Putamen
DOI: https://doi.org/10.58530/2022/5072