5071
A comparison of child and adult perceptions and experiences of having an MRI at 7T1London Collaborative Ultra high field System (LoCUS), London, UK, Kings College London, London, United Kingdom, 2Guys and St Thomas’ NHS Foundation Trust, Kings College London, London, United Kingdom, 3Perinatal Imaging and Health, Kings College London, London, United Kingdom, 4Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, Kings College London, London, United Kingdom, 5Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, Kings College London, London, United Kingdom, 6Radiology Department, Great Ormond Street Hospital for Children, London, United Kingdom, 7Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology and Neuroscience, Kings College London, London, United Kingdom, 8MR Research Collaborations, Siemens Healthcare Limited, London, United Kingdom, 9MRC Centre for Neurodevelopmental Disorders, Kings College London, London, United Kingdom
Synopsis
The aim of this study was to gather data from children on their subjective experiences when undergoing high field MRI and compare this to adult data collected with similar questionnaires. Seventeen children and twenty-six healthy adults had brain imaging at 7T. Their experiences which included: (a) acoustic noise, (b) anxiety, (c) metallic taste (d) vertigo (dizziness) and e) involuntary eye movement (nystagmus) or flashing lights were evaluated. We found that children scanned at 7T reported similar experiences to adults.
Background
The experience of adults being scanned at 7T is well documented, with reported transient effects including varying perceptions of acoustic noise, claustrophobia, nystagmus, dizziness, heating and peripheral nerve stimulation (PNS)(1–4). There is limited data regarding the experience of children(5),(6). Elucidating these experiences may play an important role in the successful scanning of children at 7T. The aim of this study was to gather data from children on their subjective experiences when undergoing high field MRI and compare this to adult data collected with similar questionnaires.Methods
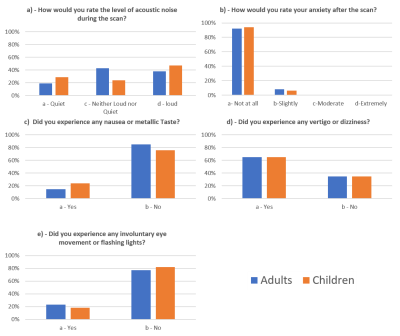
Seventeen children, a mix of healthy controls and participants with epilepsy (age range: 8-17 years, median: 11 years) and twenty-six healthy adults (age range: 25-62 years, median: 38 years), were scanned on a Magnetom Terra 7T scanner(7) (Siemens Healthcare, Erlangen, Germany) at the LoCUS MRI Unit. Due to regulatory restrictions (CE/FDA approval for scanning), participants under 30kg (66lbs)(8) were precluded. Participants within the study were scanned under local ethical approval (REMAS 8700 for adults and IRAS 243811 for children). All participants underwent a brain scan in the supine headfirst position, using either a 1TX-32RX or 8TX-32RX Nova Head Coil, and were provided with earplugs for hearing protection. Total scan duration for all participants was limited to 60 minutes, with sequences acquired in a mix of normal and first level SAR modes. Parameters for the structural sequences that were used can be seen in Figure 1. Participants experiences were evaluated immediately after the scan using a locally designed questionnaire. Children aged <11years were given a modified age-appropriate version of the questionnaire (using pictograms) to allow the child themselves to respond. All subjects were asked to consider the following experiences: (a) acoustic noise, (b) anxiety, (c) metallic taste (d) vertigo (dizziness) and (e) involuntary eye movement (nystagmus) or flashing lights. Adults and children aged 11-17 years were asked to rate acoustic noise and anxiety using Likert scales (Noise: Quiet, Neither Loud nor Quiet, Loud; Anxiety: Not at all, Slightly, Moderate, Extremely). Metallic taste, vertigo, and nystagmus were rated by a binary yes or no answer. Children aged <11 years were asked to use the same rating principles using age-appropriate language. The proportion of adult and child participants reporting each rating was calculated. Questionnaires had a ‘free comment’ section for participants to describe any further experiences they had during the scan.Results
Reported rating responses are shown in Figures 2a-e. Free text comments were given by 22 out of the 26 adults and 6 of the 17 children. The most frequent transient effect that adults mentioned was vertigo, with 22 adults commenting on this. Two adults commented on the discomfort of the head coil, whereas acoustic noise, metallic taste, and nystagmus were only mentioned by one adult participant each. One adult (with an MRI background) reported experiencing peripheral nerve stimulation. Five children responded in the free comments section, all describing their experience of feeling dizzy.Discussion
We found similar perceived experiential effects between adults and children aged ≥8years undergoing brain scans on a 7T Magnetom Terra MRI scanner. Our paediatric participants all tolerated the scans well, with no apparent difference in experience to the adult participants, though our cohort sizes were too small for formal statistical testing. Unlike for adults, where transient experience effects at 7T are well documented(1–3,6,9,10), there is limited prior data on experiences for child participants. Existing data relies on making predictions of how children will tolerate a scan from data collected at 3T(5,6). In our study, children did not report feeling any more anxious than adults (Figure 2b). This may have been influenced by all child participants having had a 3T scan at some time before their 7T scan. As such they had an idea of what to expect, but also probably lacked any pre-conceptions of expected differences about being scanned at 7T. As already noted, for regulatory reasons children less than 30kg in weight were not studied. Children also did not perceive the scanner to be any noisier than did adults (Figure 2a), or to have appreciably higher rates of reporting nystagmus, dizziness, and metallic taste (Figures 2c,2d,2e). Dizziness was commonly reported by >60% of adult and child participants using the Likert scales and was noted as the most common transient effect in both adults and children in the free comment section of the questionnaires. This effect was most commonly felt when entering the scanner. Dizziness is noted to be the effect that the magnetic field has on the vestibular system whilst moving through the static magnetic field(9,10). Our study showed that this experience is common to both adults and children and justifies the commonly recommended approach of introducing the participant into the magnet bore slowly.Conclusion
In our small study, children aged ≥8 years scanned at 7T gave similar reports of physiological effects and sensations when being positioned as our sample of adults. Whilst >60% reported dizziness, particularly on entering the scanner bore, it did not result in higher rates of anxiety than the adults or lead to scans being poorly tolerated.Acknowledgements
This work was supported by a Wellcome Trust Collaboration in science award [WT201526/Z/16/Z], by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.References
1. Rauschenberg J, Nagel AM, Ladd SC, Theysohn JM, Ladd ME, Möller HE, et al. Multicenter study of subjective acceptance during magnetic resonance imaging at 7 and 9.4 T. Invest Radiol. 2014;49(5):249–59.
2. Versluis MJ, Teeuwisse WM, Kan HE, Van Buchem MA, Webb AG, Van Osch MJ. Subject tolerance of 7 T MRI examinations. J Magn Reson Imaging. 2013;38(3):722–5.
3. Theysohn JM, Maderwald S, Kraff O, Moenninghoff C, Ladd ME, Ladd SC. Subjective acceptance of 7 Tesla MRI for human imaging. Magn Reson Mater Physics, Biol Med. 2008;21(1–2):63–72.
4. Hansson B, Markenroth Bloch K, Owman T, Nilsson M, Lätt J, Olsrud J, et al. Subjectively Reported Effects Experienced in an Actively Shielded 7T MRI: A Large-Scale Study. J Magn Reson Imaging. 2020;1–12.
5. Chou IJ, Tench CR, Gowland P, Jaspan T, Dineen RA, Evangelou N, et al. Subjective discomfort in children receiving 3 T MRI and experienced adults’ perspective on children’s tolerability of 7 T: A cross-sectional questionnaire survey. BMJ Open. 2014;4(10):1–8.
6. Veersema TJ, Ferrier CH, Eijsden P Van, Gosselaar PH, Visser F, Zwanenburg JM, et al. Seven tesla MRI improves detection of focal cortical dysplasia in patients with refractory focal epilepsy. 2017;4–10.
7. Siemens. Siemens Healthineers; Magnetom Terra [Internet]. 2021 [cited 2021 Sep 22]. Available from: https://www.siemens-healthineers.com/en-uk/magnetic-resonance-imaging/7t-mri-scanner/magnetom-terra
8. FDA; FDA clears first 7T magnetic resonance imaging device [Internet]. U.S Food & Drugs Administration. 2017 [cited 2021 Sep 22]. Available from: https://www.fda.gov/news-events/press-announcements/fda-clears-first-7t-magnetic-resonance-imaging-device
9. Theysohn JM, Kraff O, Eilers K, Andrade D, Gerwig M, Timmann D, et al. Vestibular effects of a 7 tesla MRI examination compared to 1.5 T and 0 T in healthy volunteers. PLoS One. 2014;9(3):3–10.
10. Mian OS, Li Y, Antunes A, Glover PM, Day BL. On the Vertigo Due to Static Magnetic Fields. 2013;8(10).