5054
Improving MRI in patients with Cochlear Implants (CIs)1Radiology Department, Glan Clwyd Hospital, Bodelwyddan, Wales, United Kingdom, 2Betsi Cadwaladr University Health Board, Bangor, Wales, United Kingdom, 3British Cochlear Implant Group MRI Working Group, Bradford, United Kingdom, 4Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 5Hearing Theme, NIHR Nottingham Biomedical Research Centre, Nottingham, United Kingdom, 6Hearing Sciences, School of Medicine, University of Nottingham, Nottingham, United Kingdom
Synopsis
A cochlear implant (CI) contains an implanted magnet placed under the scalp. Deaf children often receive a CI in their first year and as such are likely to need MRI in their lifetime. The presence of an implanted magnet raises safety concerns around MRI, with MRI often being avoided in favour of other imaging techniques that are less diagnostically powerful and use ionising radiation. The British Cochlear Implant Group (BCIG) MRI Working Group was assembled with the aim of collecting existing best practice and produce a set of guidance for the safe and effective clinical MR in individuals with CIs.
Motivation
A cochlear implant (CI) partially restores hearing to deaf individuals. A CI contains an implanted magnet and electronic receiver/stimulator placed under the scalp. Deaf children generally receive a CI in the first year of life and thus are likely to require post-CI-implantation MRI during their lifetime (for either clinical diagnostic or research purposes). The presence of a magnet raises safety concerns around MRI of any anatomical region in CI users, due to the risk of severe discomfort, and ultimately the displacement of the implant magnet (causing soft-tissue damage and a prolonged period of healing, during which the CI cannot be used) while in proximity to the scanner [1]. Accordingly, MRI is often avoided, in favour of other imaging techniques that are less diagnostically powerful and use ionising radiation. MRI of the head is further confounded by image distortion caused by the presence of the implant [2]. Many sites globally have a policy not to conduct MRI in patients with CIs [3]. Patients who are CI users have significant concerns about undergoing MRI in relation to their CI [4]. CI manufacturers have made design advancements to recent models of CI, with this new generation of implants with rotating magnet designs being MR conditional up to 3 T without the need for splinting or bandaging [5]. However, this has also significantly increased the heterogeneity of devices in the currently implanted population. The British Cochlear Implant Group (BCIG) MRI Working Group (comprising practitioners in audiological and radiological fields, CI manufacturers, and academics) was assembled with the aim of collating best practice and producing a set of guidelines for the safe and effective MR in individuals with CIs. This guidance aims to filter best practice down from the most experienced sites to less experienced sites, such that sites with no prior experience of scanning patients with CIs have the expertise necessary to identify which patients can undergo clinical MR with the minimum level of risk, thereby reducing unnecessary referrals of patients to more experienced departments. The guidance aims to be flexible, sustainable, clear, and succinct.Methods
Ten MRI departments around the UK and Ireland provided current working standardised operating procedures and/or protocols for conducting clinical MR in patients with CIs. All protocols were reviewed and a union of all protocols produced that contained all elements present in any one or more protocols. This was used to develop guidance for writing a site-specific protocol. Feedback was sought on this protocol from the working group. The following considerations were taken:- The need not to produce a template protocol, but guidance on producing a protocol. This was to avoid placing undue responsibility on the BCIG, to ensure longevity of the guidance, and to produce a document that addressed the significant site-based variation in information needed in a protocol.
- All model-specific information, including MR conditionality, are available from CI manufacturers’ websites, and these can change.
- The document should be as short as possible.
- At this stage, the guidance only refers to CIs, and not to Auditory Brainstem Implants (ABIs) or other auditory implantable devices.
Results
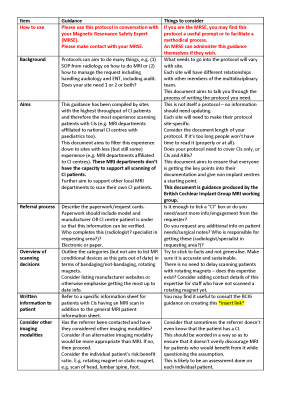
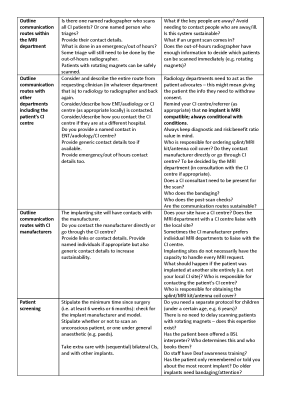
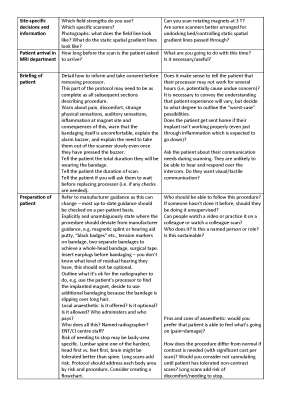
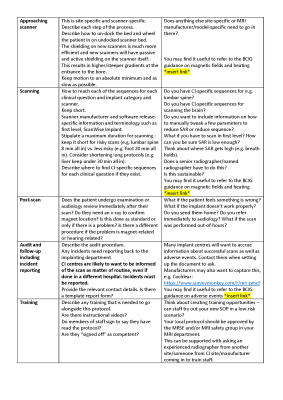
A succinct 4-page guidance for writing a protocol was produced, covering the following items: (1) How to use, (2) Background, (3) Aims, (4) Referral process, (5) Overview of scanning decisions, (6) Written information to patient, (7) Consider other imaging modalities, (8) Outline communication routes within the MRI department, (9) Outline communication routes with other departments including the patient’s CI centre, (10) Outline communication routes with CI manufacturers, (11) Patient screening, (12) Site-specific decisions and information, (13) Patient arrival in MRI department, (14) Briefing of patient, (15) Preparation of patient, (16) Approaching scanner, (17) Scanning, (18) Post-scan, (19) Audit and follow-up including incident reporting, and (20) Training. The guidance in its current draft format is given in Figures 1 to 5. We are seeking feedback on this guidance, as well as sites to volunteer in pilot testing the guidance. As such, it is imperative that it is widely publicised during the feedback stage.Discussion
There is a great need for standardised, unambiguous, evidence-led guidance for conducting clinical MRI in CI users. The next steps are to seek feedback from a wide audience prior to conducting pilot testing of the guidance in sites with little current experience of scanning patients with CIs. The BCIG MRI working group is in a position to communicate with the CI manufacturers and provide feedback on the information they publish. A common theme in many of the existing protocols was the lack of sustainability. Tasks would often fall to one named individual. The guidance can be used by MR Safety Experts (MRSE) or in combination with consulting an MRSE. In addition to the guidance on writing a protocol, the working group has produced draft guidance concerning the handling of adverse events, information to be given to patients who are CI users prior to their MRI scan and dealing with magnetic field and heat issues. Further work is needed to produce a set of CI imaging protocols, optimised for both signal and contrast of acquisition, while conforming to the SAR limitations of active implantable devices, for distribution throughout the MR community.Acknowledgements
This work was conducted within the British Cochlear Implant Group (BCIG) MRI Working Group, which comprises practitioners in audiology, ENT surgery, radiography, and medical physics, as well as representatives from cochlear implant manufacturers and higher education institutions. This research was supported by the NIHR Nottingham Biomedical Research Centre.References
[1] Walton J, et al. Otol Neurotol. 2014 Jun;35(5):821-5.
[2] Edmonson HA, et al. Radiographics. 2018 Jan-Feb;38(1):94-106.
[3] Dewey RS et al. under review.
[4] Dewey RS and Kitterick PT, Cochlear Implants Int. 2021 Jul 27:1-10.
[5] Dewey RS et al. Otol Neurotol. 2021 Jul 9. Online ahead of print.
Figures