Temperature & the Human Brain: Temperature Acquisition Strategies
1Tokai University, Hiratsuka, Japan
Synopsis
To measure brain temperature as a biomarker for detecting the thermal homeostasis and its breakdown due to trauma or cerebral infarction, or heat generation due to functional activation, we need to sharpen the MR thermometry techniques to the utmost limit. The purpose of this lecture is to discuss about the technique and the background physics of absolute and/or high-precision thermometry.
Target Audience
- Clinicians and researchers who want to have brain temperature as a new biomarker
- Physicists and engineers developing sequences and data processing techniques
Objectives
To understand the followings:- Physical principle of MR thermometry, in particular using PRF
- Factors determining temperature dependence of PRF
- MRI&S techniques applicable to thermometry
- Future directions
Introduction
It is well known that most proton (1H) magnetic resonance (MR) parameters are temperature dependent(1). These include the proton resonance frequency (PRF) or chemical shift (CS) of hydroxyl or other groups with hydrogen bonding, spin-lattice relaxation time, T1 (2-5); spin-spin relaxation time, T2 (3, 6, 7); proton density or thermal equilibrium magnetization, M0 (8, 9); and the diffusion coefficient, D (10, 11). Among these parameters, PRF can be measured independently from the other parameters, which are determined using signal amplitudes and are thus affected by each other within a limited measurement time. PRF of water molecules is thought to be the most reasonable and practical indicator because water is the natural and most abundant compound in biological bodies.There are two major approaches, MR spectroscopy (MRS) (12-17) and phase mapping (18, 19) to measure the water PRF. Although the phase mapping techniques have been widely accepted (20-22) for monitoring thermal therapies (23-26), they yield only relative temperature change with reference to a baseline temperature. To make MR-measured temperature as a biomarker(27), priority should be given to steadily improve MRS techniques.
Factors for temperature dependence of water PRF
Hydrogen bondingHydrogen bonds between the protons in the hydroxyl groups and other more electronegative atoms are primarily responsible for inducing the temperature dependence of the chemical shift of protons(28). Briefly, the electrical currents from the electronic orbitals surrounding the MR nuclei produce a weak magnetic field that opposes the external applied field (B0), effectively producing a shielding effect.(29) Consequently, the resonance frequency of the water proton decreases with the rate around -0.01ppm/oC(30). A schematic diagram of this shielding effect is depicted in Figure 1(31).
Electrolytes and dilution
We can also observe the downward shift of the MR frequency in water when univalent electrolytes are added. This phenomenon is referred to as “bond-breaking processes”(32) . When sodium or potassium ions are dissolved in water, their positive charges attract oxygen atoms in the hydroxyl group which increases the likelihood of breaking the hydrogen bonds between other water molecules.(32) The slope of the temperature change of water PRF slightly increase with ionic concentrations up to 1 M applied as shown in Fig. 2a.(33)
Bulk susceptibility variations
When a group of nuclei are in a isotropic region at temperature T, the nuclei experience a local magnetic field, Bloc(T) described elsewhere (34, 35). The rate of volume susceptibility change with temperature in an aqueous medium is negative, and is several-fold smaller (~ -0.002 ppm/oC)(34, 35) than that of the screening constant of water protons. However, it is also known that the blood oxygenation dependent susceptibility during functional activation reaches as large as 0.02 ppm(36), which corresponds to the PRF shift equivalent to 2 oC temperature change. Moreover, the susceptibility difference between completely deoxygenated and completely oxygenated red blood cells can be 0.264 ppm.(37)
pH
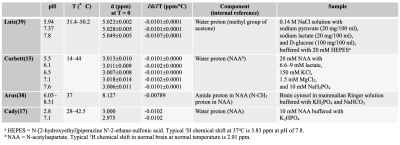
The effect of the pH on the PRF of an aqueous hydroxyl group is presently unresolved. Some of the published results are summarized in Table 1 (15, 17, 38, 39). As long as the pH is constant during the period temperature is being monitored and only relative temperature change is of interest, the effect of pH can generally be neglected. Meanwhile, we do need to consider pH effects when attempting absolute temperature measurements.
Macromolecules
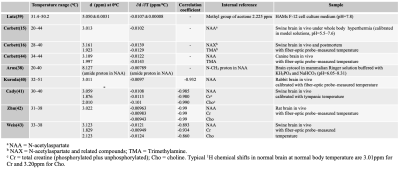
Generally, water molecules tend to cluster around large protein molecules due to hydrogen bonding, and there is exchange between these molecules and freer water in the cytosol. Averaged over the time period of observation, the hydrogen bonding causes a small increase in the water PRF as compared to that of pure water. Some representative results of temperature dependence of the proton chemical shifts in tissues obtained with internal references are summarized in Table 2 based on the previous studies (15, 16, 38-43) .
Acquisition Strategies for brain temperature measurement
Acquisition Strategies for brain temperature measurementMRS techniques with internal reference are focused here. Single voxel spectroscopy using conventional Point Resolved Spectroscopy (PRESS) has been extensively explored the temperature dependence of the water PRF, and to measure temperature at various spatially localized sites in tissue.(15, 42, 44, 45) As an internal reference in brain, a temperature-insensitive reference metabolite, such as the methyl resonance of N-acetylaspartate (NAA; ~1.98 ppm) has been preferred because of its prominence and relatively isolated chemical shift position in cerebral spectra(15, 16, 38-41, 45). The resonances from choline-containing metabolites (Cho; ~3.2 ppm) and total creatine (Cr; ~3.0 ppm) have also been used(41, 42). Signal averaging is essential for detecting reference signals from relatively low concentration (~10 mM) of these metabolites. Spectral processing techniques are also the key for quantification of the exact resonance frequencies of water and metabolite signals(41).
2D- and 3D- Magnetic Resonance Spectroscopic Imaging (MRSI) have been explored for imaging temperature.(43, 46-48). Recent technique with adiabatic selective refocusing pulse such as sLASER (semi localized adiabatic selective refocusing)(49) in conjunction with sophisticated shimming techniques such as FASTESTMAP and GRESHIM(50) has greatly improved the spectral quality good for thermometry(27).
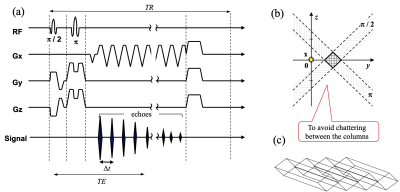
To overcome the low temporal resolution of the phase-encoding-based SI approach, echo-planar spectroscopic imaging (EPSI) (51, 52) and line-scan EPSI (LSEPSI) (40, 48, 53) have been introduced for thermometry. The latter is a combination of the echo-planar technique with column scanning that accelerates multi-voxel MRS as shown in Fig. 2, while avoiding spectral degradation due to eddy currents from the phase-encoding process. It should be noted that the EPSI and LSEPSI approaches are substantially similar to the multiple gradient-echo phase mapping technique.(54)
Other possible means
Other than the PRF approach, there has been a few attempts to use diffusion coefficient of cerebrospinal fluid (CSF) to measure brain temperature(55). Indeed, there is clear temperature dependence in diffusion coefficient in pure water(56) , we cannot deny the effect of macromolecules or other factors in the diffusivity of the water molecules in CSF.Some nuclei other than protons such as 13C,(57) 31P,(58, 59) or 59Co,(60) also demonstrate properties that may be suitable for thermometry. However, it is difficult to justify their use in the practical consequences of their lower abundance and sensitivity, and the hardware/software limitations of MR scanners.
Hyperpolarized 129Xe in a cryptophane-A cage has a temperature-dependent chemical shift of 0.29 ppm/oC.(61) Although this temperature coefficient is more than 10 times larger than that of water protons and this phenomenon is interesting as basic science, the hyperpolarization process is laborious, and clinical translation has yet to be seen.
Acknowledgements
The author thanks all his colleagues for their collaboration and contribution.References
References
1. Kuroda K, Tsutsumi S,. Chapter C: NMR tomography II: Temperature imaging by proton chemical shift. In: Miyakawa M BJ, editor. Non-invasive thermometry of the human body. Boca Raton: CRC Press Inc; 1996.
2. Lewa CzJ, A B. Proton magnetic relaxation in dehydrated normal and neoplasmic animal tissue. Bull Cancer. 1977;64:37.
3. Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1 - 100 MHz: Dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med Phys. 1984;11:425.
4. arker DL. Applications of NMR imaging in hyperthermia. IEEE Trans Biomed Eng. 1984;BME-31:161.
5. Dickinson RJ, Hall AS, Hind AJ, Young IR. Measurement of changes in tissue temperature using MR imaging. J Compt Assist Tomogr. 1986;10:468.
6. Nelson TR, Tung SM. Temperature dependence of proton relaxation times in vitro. Magn Reson Imag. 1987;5:189.
7. Kuroda K, Tsutsumi S, A S. Non-invasive temperature distribution measurement by simultaneous use of multiple NMR parameters. Trans IEICE. 1990;J73-A(8):1431.
8. Kamimura Y. Nagoya University, PhD Thesis. 1985.
9. Chen J, Daniel BL, Pauly KB. Investigation of proton density for measuring tissue temperature. J Magn Reson Imaging. 2006;23(3):430-4.
10. Hall AS, Prior MV, Hand JW, Young IR, Dickinson RJ. Observation by MR imaging of in vivo temperature changes induced by radio frequency hyperthermia
. J Compt Assist Tomogr. 1990;14:430.
11. Le Bihan D, Delannoy J, Levin RL. Temperature mapping with MR imaging of molecular diffusion imaging: Application to hyperthermia. Radiology. 1989;171:853.
12. Hall LD, Talagala Sl. Mapping of pH and temperature distribution using chemical-shift-resolved tomography. J Magn Reson,. 1985;65:501.
13. Kuroda K, Abe K, Tsutsumi S, Ishihara Y, Suzuki Y, Satoh K. Water proton magnetic resonance spectroscopic imaging. Biomed Thermol 1994;13:43-62.
14. Kuroda K, Suzuki Y, Ishihara Y, Okamoto K, Suzuki Y. Temperature mapping by water proton chemical shift obtained with 3D-MRSI - Feasibility in vivo -. Magn Reson Med. 1996;35:20-9.
15. Corbett RJT, Laptook AR, Tollefsbol G, Kim B. Validation of a noninvasive method to measure temperature in vivo using 1H NMR spectroscopy. Neurochem J. 1995;64:1224-30.
16. Corbett RJT, Laptook AR, Weatherall P. Noninvasive measurements of human brain temperature using volume-localized proton magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1997;17:363-9.
17. Cady EB, D'souga PC, Penrice J, Ann L. The estimation of local brain temperature by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 1995;33:862-7.
18. Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34:814-23.
19. Poorter JD, Wagter CD, Deene YD, Thomsen C, Stahlberg F, Achten E. Non-invasive MRI thermometry with the proton resonance frequency shift (PRF) method: in vivo results in human muscle. Magn Reson Med. 1995;33:74-81.
20. Rieke V, Vigen KK, Sommer G, Daniel BL, J.M. P, Butts K. Referenceless PRF Shift Thermometry. Magn Reson Med. 2004;51:1223-31.
21. Cline HE, Hynynen K, Schneider E, Hardy CJ, Maier SE, Watkins RD, et al. Simultaneous magnetic resonance phase and magnitude temperature maps in muscle. Magn Reson Med. 1996;35:309-15.
22. Peters RD, Hinks RS, Henkelman RM. Ex vivo tissue-type independence in proton-resonance frequency shift MR thermometry. Magn Reson Med. 1998;40:454-9.
23. Hynynen K, Freund WR, Cline HE, Chung AH, Watkins RD, Vetro JP, et al. A clinical, noninvasive, MR imaging-monitored ultrasound surgery method. Radiographics. 1996;16(1):185-95.
24. Moriaty JA, Chen JC, Purcell CM, Ang LC, Hinks RS, Peter RD, et al. MRI monitoring of interstitial microwave-induced heating and thermal lesions in rabbit brain in vivo. J Magn Reson Imag. 1998;8:128-35.
25. Kettenbach J, Silverman SG, Hata N, Kuroda K, Saiviroonporn P, Zientara GP, et al. Monitoring and visualization technique for MR-guided laser ablation in an open MR system. J Magn Reson Imag. 1998;8:933-43.
26. Kahn T, Harth T, Kiwit JCW, Schawarzmaier H-J, Wald C, Mödder U. In vivo MRI thermometry using a phase-sensitive sequence: preliminary experience during MRI-guided laser-induced interstitial thermotherapy of brain tumors. J Magn Reson Imag. 1998;8:160-4.
27. Dehkharghani S, Qiu D. MR Thermometry in Cerebrovascular Disease: Physiologic Basis, Hemodynamic Dependence, and a New Frontier in Stroke Imaging. AJNR Am J Neuroradiol. 2020;41(4):555-65.
28. Kuroda K. Non-Invasive MR thermography using water proton chemical shift. Int J Hyperthermia 2005;21(6):547-60.
29. Muller N, Reiter R. Temperature dependence of chemical shifts of protons in hydrogen bonds. J Chem Phys. 1965;42(9):3265-9.
30. Hindman J. Proton resonance shift of water in gas and liquid states. J Chem Phys. 1966; 44:4582.
31. Kuroda K. Temperature Monitoring Using Chemical Shift. In: Bottomley PA, Griffiths JR, editors. Handbook of Magnetic Resonance Spectroscopy In Vivo: MRS Theory, Practice and Applications. Chichester, UK: John Wiley & Sons Ltd; 2016. p. pp 401-14.
32. Hindman J. Nuclear magnetic resonance effects in aqueous solutions of 1-1 elecrolytes. J Chem Phy. 1962;36(4):1000-15.
33. Kuroda K, Itoh Y, Chung A, Hynynen K, Jolesz F, editors. Temperature dependence of water proton chemical shift in tissue. 4 th Meeting ISMRM; 1996; New York.
34. Poorter J. Non-invasive MRI thermometry with the proton resonance frequency method: study of susceptibility effects. Magn Reson Med. 1995;34:359-67.
35. Sprinkhuizen S, Konings M, van der Bom M, Viergever M, Bakker C, Bartels L. Temperature-induced tissue susceptibility changes lead to significant temperature errors in PRFS-based MR thermometry during thermal interventions. Magn Reson Med. 2010;64(5):360-72.
36. Kennan RP, Scanley BE, Innis RB, Gore JC. Physiological basis for BOLD MR signal changes due to neuronal stimulation: separation of blood volume and magnetic susceptibility effects. Magn Reson Med. 1998;40(6):840-6.
37. Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJ. Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T(1), T(2), T*(2), and non-Lorentzian signal behavior. Magn Reson Med. 2001;45(4):533-42.
38. Arus C, Chang YC, Baramy M. N-Acetylaspartate as an intrinsic thermometer for 1H NMR of brain slices. J Magn Reson, . 1985;63:376.
39. Lutz KW, Kuesel AC, Hull WE. A 1H-NMR method for determining temperature in cell culture perfusion systems. Magn Reson Med 1993;29:113-8.
40. Kuroda K, Takei N, Mulkern RV, Oshio K, Nakai T, Okada T, et al. Feasibility of Internally-Referenced Temperature Imaging Using Metabolite Signals. Magn Reson Med Sci. 2003;2(1):17-22.
41. Cady EB, Penrice J, Robertson NJ. Improved reproducibility of MRS regional brain thermometry by 'amplitude-weighted combination'. NMR in biomedicine. 2011;24(7):865-72.
42. Zhu M, Bashir A, Ackerman JJ, Yablonskiy DA. Improved calibration technique for in vivo proton MRS thermometry for brain temperature measurement. Magn Reson Med. 2008;60(3):536-41.
43. Weis J, Covaciu L, Rubertsson S, Allers M, Lunderquist A, Ortiz-Nieto F, et al. Phase-difference and spectroscopic imaging for monitoring of human brain temperature during cooling. Magn Reson Imaging. 2009;30(10):1505-11.
44. Corbett R, Laptook A, Weatherall P. Noninvasive measurements of human brain temperature using volume-localized proton magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1997;17:363-9.
45. Corbett RJT, Purdy PD, Laptook AR, Chaney C, Garcia D. Noninvasive measurement of brain temperature after stroke. Am J Neuroradiol. 1999;20:1851-7.
46. Hall L, Talagala S. Mapping of pH and temperature distribution using chemical-shift-resolved tomography. J Magn Reson. 1985;65:501.
47. Kuroda K, Suzuki Y, Ishihara Y, Okamoto K, Suzuk iY. Temperature mapping by water proton chemical shift obtained with 3D-MRSI - Feasibility in vivo -. Magn Reson Med. 1996;35:20-9.
48. Mulkern R, Chung A, Jolesz F, Hynynen K. Temperature monitoring of ultrasonically heated muscle with RARE chemical shift imaging. Med Phys. 1997;24:1899-906.
49. Scheenen TW, Klomp DW, Wijnen JP, Heerschap A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008;59(1):1-6.
50. Zhong X, Lyubich YM, DeVito T, Shah S, Knight-Scott J, editors. Improving In Vivo 1H-MRS with Robust Automated Shim Techniques: A Comparison Study of FASTESTMAP and GRESHIM. Proc 20th Int Soc Magn Reson Med; 2012; Melbourme, Australia.
51. Posse S, DeCarli C, LeBihan D. Three-dimensional echo-planar MR spectroscopic imaging at short echo times in the human brain. Radiology. 1994;192:733-8.
52. Kuroda K, Oshio K, Mulkern R, Panych L, Nakai T, Moriya T, et al. Temperature Mapping Using Water Proton Thermal Shift: Self-Referenced Method with Echo Planar Spectroscopic Imaging. Magn Reson Med 2000;43:220-5.
53. Oshio K, Kyriakos W, Mulkern R. Line scan echo planar spectroscopic imaging. Magn Reson Med. 2000;44(4):521-4.
54. Mulkern R, Panych L, McDannold N, Jolesz F, Hynynen K. Tissue temperature monitoring with multiple gradient echo imaging sequences. J Magn Reson Imag 1998;8:493 - 502.
55. Sparacia G, Sakai K. Temperature Measurement by Diffusion-Weighted Imaging. Magnetic resonance imaging clinics of North America. 2021;29(2):253-61.
56. Mills R. Self-diffusion in normal and heavy water in the range 1-45.deg. The Journal of Physical Chemistry. 1973;77(5):685-8.
57. Bemsk iG, Leon V, Manzo F. Temperature dependence of 13C chemical shifts in myoglobin. Acta cientifica venezolana. 1980;31(2):125-7.
58. Temussi P, Guidoni L, Ramoni C, Podo F. Temperature dependence of 31P-NMR spectra of chicken erythrocyte nucleosomes. Physiological chemistry and physics. 1979;11(5):445-52.
59. Gorenstein D, Findlay J, Momii R, Luxon B, Kar D. Temperature dependence of the 31P chemical shifts of nucleic acids. A prode of phosphate ester torsional conformations. Biochemistry. 1976;15(17):3796-803.
60. Smith N, Webb A, Ellis D, Wilmes L, Obrien W. Experimental-Verification of Theoretical in-Vivo Ultrasound Heating Using Cobalt Detected Magnetic-Resonance. Ieee T Ultrason Ferr. 1995;42(4):489-91.
61. Schilling F, Schröder L, Palaniappan K, Zapf S, Wemmer D, Pines A. MRI Thermometry Based on Encapsulated Hyperpolarized Xenon. Chem Phys Chem. 2010;11(6):3529-33.
Figures
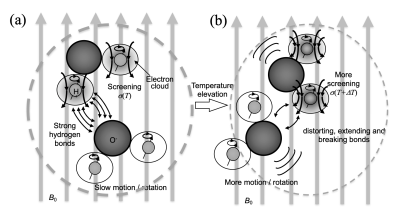
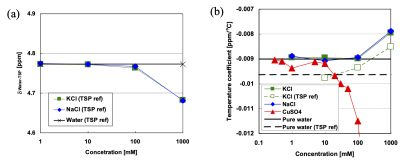
Figure 2 Effects of ions on the water PRF measured with trimethylsilylpropanoic acid (TSP) reference in electrolyte solution. Absolute values of dwater-TSP [ppm] (a) and temperature coefficients of dwater-TSP [ppm/oC] (b). (33)