Hardware Approaches for Motion Tracking
1Clinical Neuroscience, Karolinska Intitutet, Stockholm, Sweden
Synopsis
We will take a look at measuring motion in an MR scanner from a “hackers” perspective. First, we will discuss the sensors already available to us - in terms of installed hardware infrastructure. Then, we will discuss some ideas of exploiting these resources for a variety of motion detection problems. Next, we will discuss sensors. Passive sensors are typically easier to make, however, they can be limiting. This is especially true when considering the vast number of low cost sensors available, but these often aren't MRI compatible. We will look into some tricks to get around this.
Target Audience
Engineers, physicists and anyone else who likes to tinker with hardware.Purpose
To give a quick background of what has already been done in the hardware motion tracking field, discuss some exciting new directions to follow and show that augmenting the scanner hardware with new sensors is not necessarily as scary as it looks.Summary
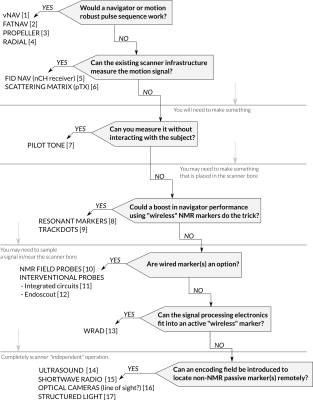
Augmenting the MR signal acquisition with sensors opens up opportunities for advanced image reconstruction. Measuring motion is of particular importance as it has a significant impact on the interpretation of the NMR spectra, and can be crucial for obtaining high quality images. During this short course we will work through some of the questions you should ask yourself when developing your own motion tracking hardware (Figure 1) and describe the rationale for some existing methods that will hopefully give you some inspiration.The first question, which you’ve probably already answered by showing interest in this course, is why you can’t solve your motion tracking problem using navigators [1,2] or motion robust pulse sequence designs [3,4]. This can be because these methods typically require a lot of scan time and can be limited in their temporal resolution. As this has nothing to do with hardware development we will skip over these methods, however, it is important to keep them in mind, because a new hardware device should outperform non-hardware methods to have a significant impact.
You don’t always have to create or construct a device, you can repurpose some of the existing scanner infrastructure. Free induction decay (FID) navigators are a fantastic example of hardware repurposing [5], where the multi-channel receive coils that enable parallel imaging are repurposed as motion detectors. The hardware used for SAR monitoring in parallel transmit capable scanners can also be repurposed to measure changes in reflected energy (scattering matrix) associated with cardiac and respiratory motion [6].
If you don’t want to rely on advanced infrastructure or it just isn’t sensitive enough for your problem you can try and augment the hardware with something new. An elegant example of this is transmitting a pilot tone, and detecting how respiration and cardiac motion affect its reception [7] during the conventional MR signal reception. The motion data is perfectly “timestamped” and synchronised with the data acquisition.
If markers are an option, you can construct small passive resonant circuits that couple to the MR scanner’s receive coil [8] and create spikes in the NMR spectra. This can be helpful for highly accelerated navigators. For rigid body motion detection, more than three markers are required. A correspondence problem then needs to be solved in order to uniquely identify each marker in each of a series of spectra. Small capsules filled with acetic acid (off-resonance) can also be used [9], in this case the correspondence problem is solved by taking advantage of the “spatial encoding” of a multi channel receive coil (“repurposing”). You may be noticing a trend, things are generally easier when you digitise signals using the MR scanner.
This approach can be taken one step further with wired markers that can be individually excited and sampled using either the MR hardware or your very own custom built receive/transmit chain that runs in parallel to the imaging experiment. This increases hardware complexity, however, it enables much more freedom in navigator design and very high SNR. In some cases, regular sequences can be made self-navigating [10]. These sensors can also reveal field dynamics at high temporal resolutions (field cameras).
Another advantage of wired probes is the ability to move the signal processing electronics far away from the imaging field of view. Most interventional procedures (iMRI) are compatible with wired probes - although special care should be taken when running longer cables within the scanner. One proposed solution to this is the use of photovoltaics to power an integrated circuit through an -as long as you like- optical cable. The chip is then capable of transmitting information (local NMR frequency) back by modulating the current in a small microelectromechanical (MEMs) mirror that deforms under the forces caused by interactions with the static magnetic field [11].
All the solutions discussed thus far are based on NMR, which is sensitive to both motion and field dynamics. An alternative approach of gaining spatial information from the scanners gradient fields is by detecting their rate of change with pickup coils [12]. In this case the signals are of a much lower frequency and straightforward to process with embedded electronics. This opens the opportunity to build small battery-powered digitisers that can sample sensors near the FOV and transmit the signals using low power 2.4 GHz radios [13]. It does not take a huge leap to include a wide range of commercially available sensors (accelerometers and angular rate sensors), however, the need for a battery and wireless communication limit the minimum size the device/marker can have.
One can envisage smaller passive markers that can be detected by some other means, independent of the scanner’s operation. An interesting new approach is to use a short-wave radio “reflector” that interacts with locally transmitted (~1 MHz) signals [14]. The way the “reflected” signal interacts with a set of gradiometer receive coils is unique for a specific pose of the miniaturised reflector. If you can see the object you're interested in tracking, optical markers make a lot of sense and offer some of the best tracking performance using technology from an already well established field [15]. More recently structured light has been employed to track subject motion without the use of markers [16].For organ tracking multimodal imaging strategies can be employed where a faster imaging modality can be paired with MR to perform motion tracking tasks, such as ultrasound [17].
Acknowledgements
The Karolinska Sjukhuset MR physics group.References
1. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. 2012;68: 389–399.
2. Gallichan D, Marques JP, Gruetter R. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Magn Reson Med. 2016;75: 1030–1039.
3. Pipe JG. Motion correction with PROPELLER MRI: Application to head motion and free-breathing cardiac imaging. Magn Reson Med. 1999;42: 963–969.
4. Welch EB, Rossman PJ, Felmlee JP, Manduca A. Self-navigated motion correction using moments of spatial projections in radial MRI. Magn Reson Med. 2004;52: 337–345.
5. Kober T, Marques JP, Gruetter R, Krueger G. Head motion detection using FID navigators. Magn Reson Med. 2011;66: 135–143.
6. Jaeschke SHF, Robson MD, Hess AT. Scattering matrix imaging pulse design for real-time respiration and cardiac motion monitoring. Magn Reson Med. 2019;82: 2169–2177.
7. Vahle T, Bacher M, Rigie D, Fenchel M, Speier P, Bollenbeck J, et al. Respiratory Motion Detection and Correction for MR Using the Pilot Tone: Applications for MR and Simultaneous PET/MR Examinations. Invest Radiol. 2020;55: 153–159.
8. Ooi MB, Aksoy M, Maclaren J, Watkins RD, Bammer R. Prospective motion correction using inductively coupled wireless RF coils. Magn Reson Med. 2013;70: 639–647.
9. Jorge J, Gretsch F, Gallichan D, Marques JP. Tracking discrete off-resonance markers with three spokes (trackDOTS) for compensation of head motion and B0 perturbations: Accuracy and performance in anatomical imaging. Magn Reson Med. 2018;79: 160–171.
10. Aranovitch A, Haeberlin M, Gross S, Dietrich BE, Wilm BJ, Brunner DO, et al. Prospective motion correction with NMR markers using only native sequence elements. Magn Reson Med. 2018;79: 2046–2056.
11. Kouhani MHM, Camli B, Cakaci AU, Kusakci E, Sarioglu B, Dundar G, et al. Integrated Silicon Photovoltaics on CMOS With MEMS Module for Catheter Tracking. J Lightwave Technol. 2015;33: 3426–3432.
12. Pan L, Valdeig S, Kägebein U, Qing K, Fetics B, Roth A, et al. Integration and evaluation of a gradient-based needle navigation system for percutaneous MR-guided interventions. PLoS One. 2020;15: e0236295.
13. van Niekerk A, Meintjes E, van der Kouwe A. A wireless radio frequency triggered acquisition device (WRAD) for self-synchronised measurements of the rate of change of the MRI gradient vector field for motion tracking. IEEE Trans Med Imaging. 2019.
14. Christoph Michael Schildknecht, David Otto Brunner, Thomas Schmid, and Klaas Paul Pruessmann. Design considerations for short-wave motion tracking. [4293] ISMRM 2020, Sydney. Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zurich, Switzerland; Available: https://index.mirasmart.com/ISMRM2020/PDFfiles/4293.html
15. Zaitsev M, Dold C, Sakas G, Hennig J, Speck O. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. Neuroimage. 2006;31: 1038–1050.
16. Frost R, Wighton P, Karahanoğlu FI, Robertson RL, Grant PE, Fischl B, et al. Markerless high-frequency prospective motion correction for neuroanatomical MRI. Magn Reson Med. 2019. doi:10.1002/mrm.27705
17. Bour P, Ozenne V, Marquet F, Denis de Senneville B, Dumont E, Quesson B. Real-time 3D ultrasound based motion tracking for the treatment of mobile organs with MR-guided high-intensity focused ultrasound. Int J Hyperthermia. 2018;34: 1225–1235.